It:Azithromycin
| Azithromycin | ||||
|---|---|---|---|---|
| Structure | ||||
| ||||
| 3D structure | ||||
| General | ||||
| Systematic name | 9-deoxy-9a-aza-9a-methyl-9a- homoerythromycin A | |||
| Synonyms | Zithromax Zmax Sumamed Aztrin | |||
| Molecular formula | C38H72N2O12 | |||
| SMILES | CCC(O1)[C@@](C)(O)[C@H](O)[C@@H] (C)N(C)CC[C@H](C)[C@@](O)(C)[C@H] (OC3OC(C)CC(N(C)C)[C@H]3O)C(C)C (O[C@@H]2OC(C)[C@H](O)[C@@](OC) (C)C2)C(C)C1=O | |||
| Molar mass | 748.88 g/mol | |||
| Appearance | Mottled pink tablets | |||
| CAS number | 83905-01-5 | |||
| ATC Code | J01 FA10 | |||
| Pharmacokinetic data | ||||
| Bioavailability | 38% for 250mg capsules | |||
| Metabolism | hepatic | |||
| Half life | 68 hours | |||
| Excretion | Biliary, Renal | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references] | ||||
Azithromycin is one of the world's biggest selling antibiotics, commonly used in the treatment of tonsillitis, bronchitis, pneumonia and many ear and throat infections. It is also an effective treatment for chlamydia. Coming in the form of a capsule that can be taken orally in many cases it is prefered to other treatments due to the ease of administration.
History
A team of researchers at the Croation laboratory Plivaled by Dr Slobodan Dokic, discovered azithromycin in 1980. It was patented in 1981, and was later discovered by scientists at Pfizer while going through patent documents. In 1986 Pliva and Pfizer signed an agreement which gave Pfizer rights for the sale of azithromycin in Western Europe and the USA. Azithromycin was brought on to the market in Central and Eastern Europe under the brand names of Sumamed by Pliva in 1988, and Zithromax by Pfizer in 1991.
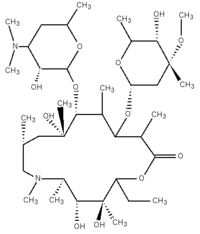
Structure
Azithromycin is derived from erythromycin but differs from erythromycin by the inclusion of a methyl substituted nitrogen in a 15-membered lactone ring.
Mode of action
Azithromycin works by binding to the active site of bacterial ribosomes, this prevents the translation (the "decoding" of a of mRNA to produce a polypeptide chain) and hence the synthesis of proteins, however the synthesis of the bacteria's DNA is not affected.
Pharmacokinetics
Unlike its parent compound, erythromycin, azithromycin is stable to acids and can therefore be taken in the form of an oral capsule with no fear of stomach acid breaking it down. It is absorbed easily into most tissues, due to its high solubility in lipids, and is carried in particularly high concentrations in phagocytes (cells of the immune system that ingests foreign matter). As the phagocytes collect in large numbers at the site of an infection this means that the antibiotic is present exactly where in the body it is needed.
Treatment of chlamydia
Azithromycin is the antibiotic of choice for the treatment of chlamydia due to its unusual pharmokinetic properties. its long half-life means that it remains in cells and tissues at an anti-chlamydial levels for far longer than other simmilarly working antibiotics, meaning a single dose of azithromycin can have the same effect as repeated doses of other drugs. This is particularly useful when a patient may be unlikely to complete a longer couse of antibiotics.
