It:Aspirin
Aspirin
It is one of the best known aromatic acetates, which is also called acetylsalicylic acid.

Synthesis
It is prepared by the esterification of the phenolic hydroxyl group of salicylic acid. Kolbe-Schmitt process is used industrially to prepare salicylic acid, a precursor in making aspirin.
How aspirin works
Aspirin: whilst the acid itself is not very soluble in water, the sodium salt is much more soluble (soluble aspirin is actually the sodium or calcium salt of ‘normal’ aspirin). Conversely, if the pH of a solution is lowered, the amount of the acidic form present increases, and the solubility decreases. In the acidic environment of the stomach (pH around 1-2), soluble aspirin will be precipitate out of solution.
The enzyme cyclooxygenase is an important target for medical chemists. Inhibiting prostaglandin (PG) synthesis can bring about a reduction of inflammation and pain. In fact, this is how aspirin works. It was not, of course, designed to work that way and its mode of action was discovered decades after its use began. There is a price to pay for such a useful drug. PGs also control acid secretion in the stomach and aspirin inhibits their synthesis there too so stomach ulceration can result.
Further synthesis
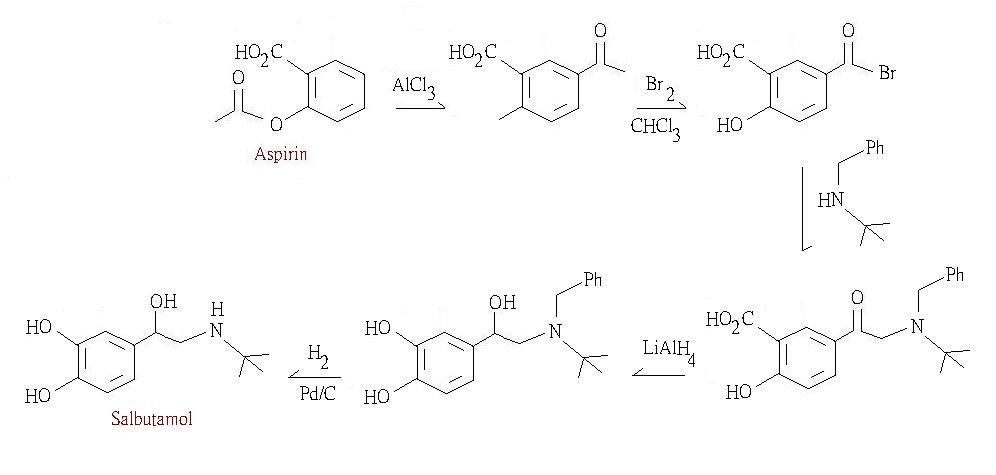
Aspirin can be used to make salbutamol, which is an anti-asthma drug works by dilating the air passages of the lungs, releasing the constriction that characterizes the disease.