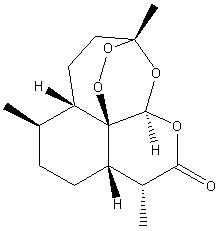
It:Artemisinin
| 2D structure of Artemisinin | ||||
|---|---|---|---|---|
| ||||
| 3D structure of Artemisinin | ||||
| Molecular weight | 282.332 g/mol | |||
| Molecular Formula | C15H22O5 | |||
| SMILES |
C[C@@H]1CC[C@]([C@H]3C)([H])[C@]2(OO4)[C@]([H])1CC[C@@] (C)4O[C@]([H])2OC3=O | |||
| CA index name | 3,12-Epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10(3H)-one,
octahydro-3,6,9-trimethyl-, (3R,5aS,6R,8aS,9R,12S,12aR)- (9CI) | |||
| Other names |
(+)-Arteannuin; (+)-Artemisinin; (+)-Qinghaosu; Arteannuin; Artemef; Artemisine; Artemisinin; Artemisinine; Huanghuahaosu; NSC 369397; QHS; Qing Hau Sau; Qing Hau Su; Qinghaosu; Qinghosu | |||
| Boiling point | 389.9±42.0 °C | |||
| Density | 1.24±0.1 g/cm3 | |||
| Enthalpy of vaporization | 63.93±3.0 kJ/mol | |||
| Flash point | 172.0±27.9 °C | |||
Artemisinin is a drug that is used to treat malaria. This compound is obtained from the leaves of the shrub ‘Artemesia annua’, which is cultivated in China [1]. Artemisinin affects the malaria parasite, which is responsible for the malaria disease in humans. The drug has no significant side effects and reduces the malarial fever.
The information in the box on the right (containing the 2d structure, 3d structure, molecular weight etc...) was obtained from SciFinder Scholar [ref 2].
Effectiveness of the drug
Artemisia has a great potential for being an effective drug to treat malaria, which is a disease that affects more than 300 million people a year (ref 3). Malaria kills about one million people a year (mainly young children), in sub-Saharan Africa and south Asia. Clinical trials in some countries (such as India, Indonesia, and Vietnam) have that over 90% of patients have recovered from malaria, when treated by using Artemisinin-based Combination Therapies (ACTs).
History
The Artemisia plant has been used in Chinese medicine for over a thousand years, but only in the 1960’s when military scientists in China started to analyse the large variety of Chinese traditional medicines, to find a substance that will protect the Chinese military from the malaria disease. The researchers were able to extract a chemical known as Artemisinin, which had the desired anti-malarial properties. The step from the 1960’s was to modify Artemisinin to become more potent against malaria, because the malaria parasite can evolve (by natural selection) to survive in the presence of Artemisinin. This has lead to scientists modifying the groups in Artemisinin, to produce a more potent drug.
Proposed mechanism of attacking the malarial parasite
The mechanism of how Artemisinin kills the malaria parasite is not well known. Theories have been proposed (ref 4-proposed mechanisms of how Artemisinin kills the malaria parasite ), but it had been difficult to develop further as not enough evidence have been obtained.
One theory states that Artemisinin does not affect the malaria parasite as a whole intact molecule, but via a species of a transition state (having a fleeting existence) that is formed after the cleaving of the peroxy bond (O-O bond). Then this species attacks the malaria parasite.
Another theory states that the anti-malarial properties of Artemisinin are due to the direct O2 transfer from a hydroperoxide ROOH group (from the ring-opening in Artemisinin). Then the reactive oxygen centered radicals formed from ROOH, attacks the haem group in the malaria parasite’s blood (red blood cells), which should kill the parasites.
More research is required to figure out the exact mechanism of how Artemisinin affects the malaria parasite.
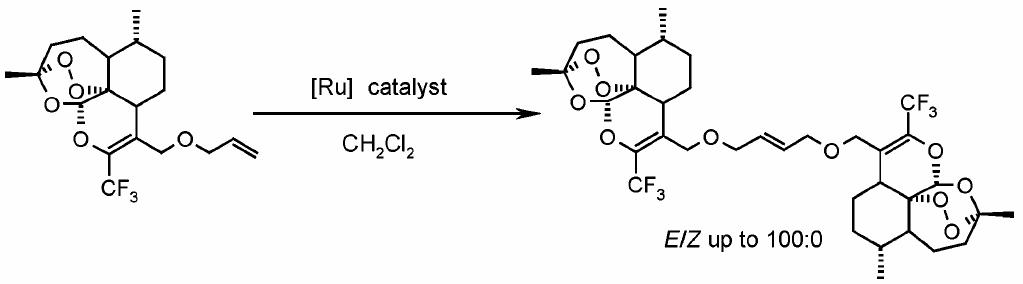
Synthesis of Artemisinin derived dimers
Artemisinin derived dimers can be synthesised by a self-cross metathesis reaction (ref 5- article on metathesis reaction ). The dimers are prepared with ruthenium catalysts, and this catalyst does not cleave open the O-O peroxy bond.
Therefore the peroxide bond can still form oxygen centered radicals (to kill malaria parasites) when in the dimer form. The peroxide bond is also toxic to tumor cells in cancer diseases. The peroxide bond is more effective against tumor cells, when in the dimer form of Artemisinin.
The self-cross metathesis reaction for the preparation of Artemisinin derived dimers had been founded upon from the work of the olefin (alkene) metathesis reaction of the past several years. Ruthenium based catalysts are used because of their stability, reactivity and functional group tolerance.
References
1) http://www.medterms.com/script/main/art.asp?articlekey=24010
2) SciFinder Scholar
3) Economist 11/20/2004, Vol. 373 Issue 8402, p81
4) proposed mechanisms of how Artemisinin kills the malaria parasite