It:Amphidinolide T1
Introduction: Amphidinolides
The amphidinolides are a group of very scarce, naturally occurring compounds found in marine microalga dinoflagellates (genus: Amphidinium). These microorganisms live in the inner tissue of the Okinawan flatworm (a Japanese flatworm, genus: Amphiscolops) and take part in a symbiotic relationship with its host. Since the initial discovery of the amphidinolides A-D by Jun’ichi Kobayashi and his co-workers in the 1980’s, further amphidinolides E-Y have been discovered by performing the extraction process on different species of the Amphidinium genus.1
The amphidinolides consist of a highly oxygenated macrolactone of varying ring sizes. Many of the amphidinolides express potent cytotoxicity, which makes them potentially effective anti-cancer fighting compounds, as they would have the ability to attack various cancer cell lines.2
The amphidinolide T class has received particular attention since it was discovered in 20003. There are five sub-groups of amphidinolide T: T1, T2, T3, T4 and T5. T1-T5 all contain 7 stereocentres apart from T2, which has 8.1
Amphidinolide T1
Amphidinolide T1 is extracted from the Y-56 strain of the marine dinoflagellate Amphidinium. Its structure consists of a 19-membered macrolide possessing a tetrahydrofuran ring, an exo-methylene, three branched methyls, a ketone, and a hydroxyl group.4
| Structure of Amphidinolide T1 | ||||
|---|---|---|---|---|
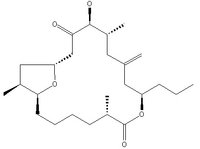
| ||||
| 3D structure | ||||
| General Properties | ||||
| Chemical Name | (+)-amphidinolide T1 | |||
| Systematic name | 14-hydroxy-6,13,19-trimethyl-11-
methylene-9-propyl-8,20-dioxa-bicyclo [15.2.1]eicosane-7,15-dione | |||
| Molecular formula | C25H42O5 | |||
| SMILES | O[C@@H]([C@@H](CC(C[C@H](O2)CCC)
=C)C)C(C[C@]1([H])O[C@@]([H])(CCCC[C@H] (C)C2=O)[C@@H](C)C1)=O | |||
| Molar mass | 422.60 g/mol | |||
| Beilstein Registry number | 8521136 | |||
| Type of Substance | heterocyclic | |||
| CAS number | ||||
| Physical Properties | ||||
| Melting point | 63-66°C | |||
| Boiling point | ||||
| Basicity (pK) | ||||
| Crystal Phase | ||||
| Crystal System | monoclinic | |||
| Crystal Density | 1.147g/cm3 | |||
| Temperature | -160C | |||
| Hazards | ||||
| Main hazards | cytotoxicity | |||
| Analytical data | ||||
| Spectral data | NMR, UV, IR, MS | |||
| Related compounds | ||||
| Other Subgroups | Amphidinolide T2, Amphidinolide T3,
Amphidinolide T4 & Amphidinolide T5 | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references] | ||||
Synthesis
Fürster’s Synthesis – Utilising a late stage intermediate to produce the product
A key intermediate was produced from three separate compounds that was fundamental to the overall synthesis strategy. This intermediate was then used to produce Amphidinolide T1 and could also be used the T3, T4 and T5 compounds of the amphidinolide family.
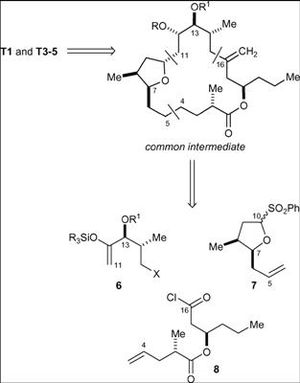
Once the intermediate had been produced then a sequence of three steps was required to produce the desired product. This involved the removal of the silyl protective group, achieved via the aid of a flurosilicate reagent, the oxidation of the alcohol to the ketone on C-12 and the deprotection of the MOM ether by exposure to acidic Dowex resin.
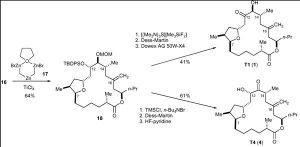
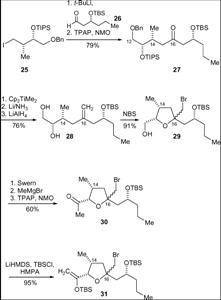
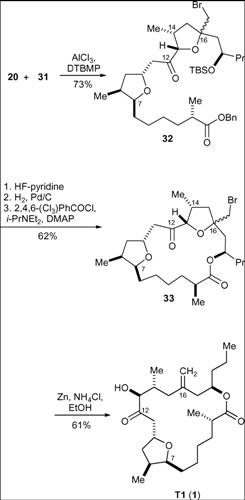
Ghosh’s Synthesis – Ring Formation via Macrolactonisation
This synthesis strategy is based on producing two fragments of similar complexity and then combining them via Fragment coupling to provide the desired final product.
Scheme 1 involves the production of the target sulfone and Scheme 2 provides the sily enol ether product. These two compounds were subjected to a Lewis acid-mediated alkylation reaction. The TBS and benzyl groups were removed and macrolactonisation followed. Zinc was then used to perform a reductive cleavage of the product of the former process and the desired product was thus achieved.
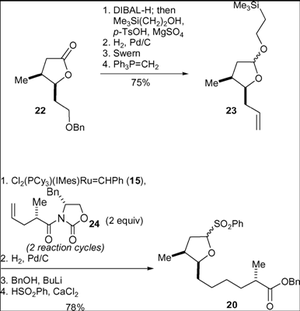
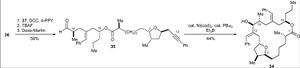
Jamison’s Synthesis – Usage of Nickel-catalysed reductive coupling reactions of alkynes
In this synthesis a carboxylic acid (36) and a homo allylic alcohol (37) were produced independently and then combined to follow a pathway to the final product. Compound 36 was synthesised from lactol. Compound 37 was produced by an alkyne-epoxide fragment coupling reaction of the alkyne (38) and the epoxide (39)

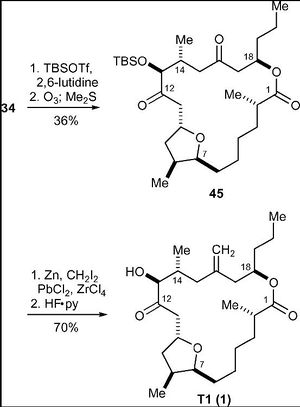
Compounds 36 and 37 were coupled via a DCC-mediated esterification reaction, followed by removal of the TBS group and an oxidation reaction. A reductive macrocyclisation reaction yielded compound 34.
In the penultimate step, TBS protection of the hydroxyl group followed by ozonolysis then produced the diketone (45). The final step involved selective methylenation followed by the removal of the TBS protective group, to produce Amphidinolide T1.
References
1. Elizabeth A. Colby, Timothy F. Jamison, A comparative analysis of the total syntheses of the amphidinolide T natural products, Org. Biomol. Chem., 2005, 3, 2675-2684
2. Elizabeth A. Colby, Karen C. O'Brien, and Timothy F. Jamison, Total Syntheses of Amphidinolides T1 and T4 via Catalytic, Stereoselective, Reductive Macrocyclizations, J. Am. Chem. Soc., 2005, 127, 4297 – 4307, DOI:10.1021/ja042733f
3. Masashi Tsuda, Tetsuya Endo, and Jun'ichi Kobayashi, Amphidinolide T, Novel 19-Membered Macrolide from Marine Dinoflagellate Amphidinium sp., J. Org. Chem., 2000, 65, 1349 – 1352, DOI:10.1021/jo991393r
4. Takaaki Kubota, Tetsuya Endo, Masashi Tsuda, Motoo Shiro and Jun'ichi Kobayashi, Amphidinolide T5, a new 19-membered macrolide from a dinoflagellate and X-ray structure of amphidinolide T1, Tetrahedron, 2001, 57, 6175-6179, DOI:10.1016/S0040-4020(01)00591-9
