It:Acrylamide
| Acrylamide | ||||
|---|---|---|---|---|
| ||||
| 3D structure | ||||
| General | ||||
| Systematic name | 2-Propenamide | |||
| Synonyms | acrylic amide, ethylene carboxamide, 2-propenamide, propenoic acid amide, vinyl amide, propenamide, acrylamide monomer | |||
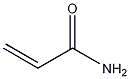
| Molecular formula | C3H5NO | |||
| SMILES | C=CC(=O)N | |||
| Molar mass | 71.08 g/mol | |||
| Appearance | White Crystaline chunks | |||
| Beilstein Registry number | 605349 | |||
| CAS number | 79-06-1 | |||
| Properties | ||||
| phase] | 1.13 g/cm³ | |||
| water] | 204 g/100 ml (25°C) | |||
| Other solvents | Ethanol, ether, chloroform | |||
| Melting point | 84.5°C | |||
| Boiling point | 125°C at 25mm Hg | |||
| Basicity | ? | |||
| Structure | ||||
| Crystal structure | ||||
| Dipole moment | ? D | |||
| Hazards | ||||
| MSDS | External MSDS | |||
| Main hazards | ||||
| NFPA 704 | ||||
| Flash point | 138 °C | |||
| R statement | R45, R46, R20/21, R25, R36/38, R43, R48/23/24/25, R62 | |||
| S statement | S53, S45 | |||
| RTECS number | ||||
| Supplementary data page | ||||
| Structure and properties | ||||
| Thermodynamic data | ||||
| Spectral data | UV, IR, NMR, MS | |||
| Related compounds | ||||
| Related compounds | aspararagine - precursor | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references] | ||||
Acrylamide is an important industrial chemical which has been in use since the mid 1950's. The neurotoxicity and carcenogenic effects are well known in humans from occupational and accidental exposure. In addition, experimental studies in animals have shown its genotoxic, reproductive and carcenogenic properties.
In 2002 it was found that high levels of acrylamide formed during the frying or baking but not the boiling of food. This caused a media frenzy at the time, which has since subsided.
Uses
Acrylamide is used to synthesize polyacrylamides which are used for water - soluble thickeners. These water soluble thickners find application in:
- Papermaking
- Wastewater treatment as floculating agents for clarifying drinking water
- Gel electrophoresis
- Ore processing
- The manufacture of press fabrics
- The manufacture of dyes
- The manufacture of other monomers
Effects of acrylamide on humans
Acrylamide as a monomer is a potent neurotoxin and exposure to large doses can cause damage to the male reproductive glands. Direct exposure to pure acrylamide by skin absorption, inhalation, or eye contact irritates the exposed mucous membranes, and can also cause sweating, nausea, urinary incontinence, speech disorders, myalgia, paresthesia, numbness, and weakened legs and hands. There is also currently speculation that acrylamide may be carcinogenic although there is no conclusive evidence of this as it is almost impossible to make up a control group of people who have not consumed acrylamide, due to the fact that it is present in most food sources.
Sources of acrylamide consumed by humans
Acrylamide is formed during the frying, baking, grilling and microwaving, but not boiling, of foods containing asparagine. Asparagine is a non essential amino acid found in high concentrations in starchy foods such as potatoes, wheat and coffee. Asparagine is also found in dairy products, beef, poultry, meat and eggs. On heating asparagine above 120°C it reacts with reducing sugars such as fructose and glucose to produce acrylamide via the Maillard reaction. Thus foods such as bread, which has been baked, chips and crisps, which have been fried, and coffee, the coffee beans have been roasted, all contain high levels of acrylamide.
Acrylamide is also found in olives and prune juice but is produced via a different mechanism. Smoking also produces large amounts of acrylamide.
It has also been suggested that the herbicide glycophosphate, marketed as Roundup, can break down via enviromental pathways to produce acrylamide.
Prevention and control of acrylamide levels in food
Current experiments on various foods have shown a number of ways to reduce acrylamide levels. One of these methods is to use the enzyme asparaginase to remove the asparagine precursor to acrylamide prior to heating, which is only really applicable to food products manufactured from liquidised or slurried materials. Other methods are to control plant growth, plant breeding to select particular plant varieties with beneficial genes, control storage factors affecting sugar concentrations in potatoes, pre-treatment of potato pieces by soaking or blanching, and prolonging yeast fermentation time in breadmaking. Other possibilities include adding amino acids or acidic compounds to food in order to compete with the asparagine in the Malliard reaction. Also altering processing conditions such as lowering the frying temperature will help to reduce the concentration of acrylamide in food.
Metabolism
When orally consumed, by rodents, acrylamide is rapidly absorbed from the gastrointestinal tract and is widely distributed to the tissues (this is most likely to be the case for humans as well as acrylamide has been found in human breast milk). Acrylamide is metabolised by the enzyme CYP2E1 in a catalytic reaction to form a chemically reactive epoxide, glycidamide. An alternate pathway for metabolism of acrylamide is conjugation with glutathione.
Acrylamide and its metabolites are rapidly eliminated in urine, mainly as mercapturic acid conjugates of acrylamide and glycidamide.
Glycidamide is much more reactive than acrylamide with DNA and forms purine base adducts via CYP2E1-mediated oxidation. These DNA adducts can be found in liver, lung, testis, leukocytes, and kidney of mice and in liver, thyroid, testis, mammary gland, bone marrow, leukocytes, and brain of rats treated with either acrylamide or glycidamide.
Studies of adduct loss from DNA showed that spontaneous toxin removal, as opposed to active repair, is operative.
References
- http://ptcl.chem.ox.ac.uk/MSDS/AC/acrylamide.html
- http://en.wikipedia.org/wiki/Acrylamide
- http://www.who.int/ipcs/food/jecfa/summaries/summary_report_64_final.pdf
- http://en.wikipedia.org/wiki/Asparagine
- http://www.aist.go.jp/RIODB/SDBS/cgi-bin/direct_frame_top.cgi?lang=eng
- http://pubs.acs.org/cgi-bin/article.cgi/jafcau/2005/53/i26/pdf/jf051197n.pdf
