It:Acrolein
| Acrolein | |
|---|---|
| |
| General [1] | |
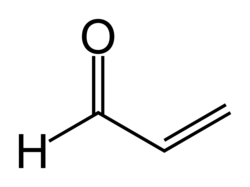
| Systematic name | 2-propenal |
| Other Names | Prop-2-en-1-al 2-propene-1-one Propenal Acraldehyde Acrylic Aldehyde Allyl Aldehyde Ethylene aldehyde acrylaldehyde Aqualin Magnacide H NSC 8819 and many more... |
| Molecular Formula | C3H4O |
| Molar Mass | 56.06 g/mol |
| CAS Number | 107-02-8 |
| Properties | |
| Melting Point | -87°C |
| Boiling Poing | 52.7°C |
| Appearance | Colorless liquid |
| Vapor Density | 1.94 (where air = 1) |
| Vapour Pressure | 286 mbar at 20°C |
| Auto-ignition Temperature | 233°C |
General
Acrolein, the simplest unsaturated aldehyde, is a colourless volatile liquid that burns easily with an acrid smell. It is soluble in water, alcohol and ether.
It is formed as the long chain fatty acids in meat break down when heated to 280°C, the glycerol molecule loses two molecules of water and hence forming acrolein. The acrid smell is responsible for the flavour of barbecue food. The process of heating and partially decomposition of sucrose lead to the characteristic smell of caramel. [4] Small amounts of acrolein may be found in cooking oils and roasted coffee.
Acrolein readily polymerizes exothermically, especially when it is in contact with strong acids or bases. Dimerization may occur at temperature higher than 150°C. [2]
cn205 10:50, 7 November 2006
Hazards
Acrolein can be formed from the breakdown of pollutants found in the atmosphere, as well as from burning tobacco or gasoline. It is found to have major connections between tobacco cigarettes and the risk of lung caner. However, it has no direct information to prove that acrolein is carcinogenic due to the lack of human data.
When acrolein is injected directly into the embryo tissue of rats and is found to cause birth defects; but it does not affect the number of pregnancies. However, effects of acrolein in humans on reproductive or developmental have not yet been proved. [3]
Acrolein is:
- Flammable
- Highly toxic if swallowed and by inhalation (inhalation exposure to high levels such as 10 ppm may be fatal
- Severe eye irritation or causes burns to the eye
- Skin exposure causes serious damage
- Poisonous at low ppm concentrations; immediately dangerous to life at 2 ppm [2]
Uses
Acrolein is used to make other chemicals and pesticides.
References
1. http://physchem.ox.ac.uk/MSDS/AC/acrolein.html
2. http://www.chemicalland21.com/industrialchem/organic/ACROLEIN.htm
3. http://www.lakes-environmental.com/toxic/ACROLEIN.HTML
4. Atkins’ Molecules 2nd ed., Peter Atkins, CUP, 2003, pages 140-141
