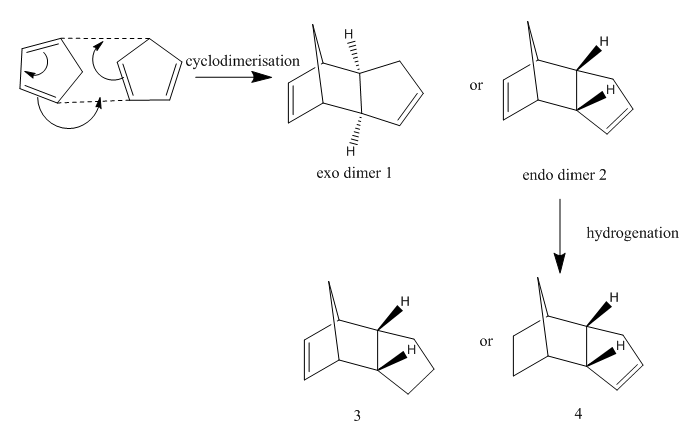
It07:wilkinson
| It07:wilkinson | |||
|---|---|---|---|
 | |||
| 3D shape | |||
| General | |||
| Systematic name | chlorotris(triphenylphosphine)rhodium(I) | ||
| Other names | Rhodium(I) tris-
(triphenylphosphine) chloride, Wilkinson’s catalyst, Tris(triphenylphosphine)- rhodium chloride | ||
| Molecular formula | C54H45ClP3Rh | ||
| Molar mass | 925.22 g/mol-1 | ||
| CAS number | 14694-95-2 | ||
| Properties | |||
| Melting point | 245-250 °C | ||
| Appearance | Red Crystalline Solid | ||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |||
Wilkinson's Catalyst
Wilkinson's catalyst is the common name for chlorotris(triphenylphosphine)rhodium(I). It is a chemical compound with the formula RhCl(PPh3)3, where Ph represents phenyl group. Wilkinson's Catalyst is a rhodium metal complex with three large phosphine ligands coordinated to the metal centre, Rh(PPh3)3Cl.[1]Its use was popularlised by organometallic chemist, Sir Geoffrey Wilkinson (1973 Nobel Laureate) and it was named afer him.
History of Geoffrey Wilkinson[2]

Sir Geoffrey Wilkinson (14 July 1921 - 26 September 1996) was a British inorganic chemist, who shared the Nobel Prize for Chemistry (1973) with Ernst Fischer. Their pioneering work was in the chemistry of the organometallic. The work had a wide effect on inorganic chemistry and started the field of organometallic chemistry.
Wilkinson studied at Imperial College with a Royal Scholarship and graduated in 1941. After his study, Wilkinson worked with Atomic Energy Project in Canada (1943-1946). He held numerous posts in North America; University of California at Berkeley (1946-50), Massachusetts Institute of Technology (1950-51, Harvard University (1951-56). He returned to Imperial College and was appointed to the chair of Inorganic Chemistry in June 1955 and became a professor in 1988. Wilkinson was knighted in 1976. He also co-wrote a text book called Advanced Inorganic Chemistry (1962) with F. A. Cotton. At Imperial he worked on the complexes of transition metals.
An organometallic molecule consists of a metal atom sandwiched between carbon rings. The synthetic compound Wilkinson and his partner were investigating was called ferrocene which has a single iron atom sandwiched between two five-sided carbon rings; later on, there were many other variations created with oher metals and four-, six-, seven-, and eight-membered carbon rings.
One of his chemical discoveries was named after him, Wilkinson's Catalyst. This is an organometallic compound which activates small organic molecules so that the bond-breaking and bond-formation pathways are readily accessible.
Synthesis
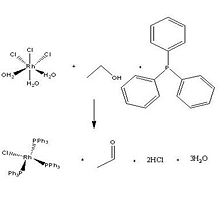
The Wilkinson's Catalyst is a square planar, 16-electron complex. It is usually synthesised by a reaction of rhodium trichloride with triphenylphosphine. This synthesis is conducted in refluxing ethanol, which serves as the reducing agent.[5]
RhCl3(H2O)3 + CH3CH2OH + 3 PPh3 → RhCl(PPh3)3 + CH3CHO + 2 HCl + 3 H2O
Applications
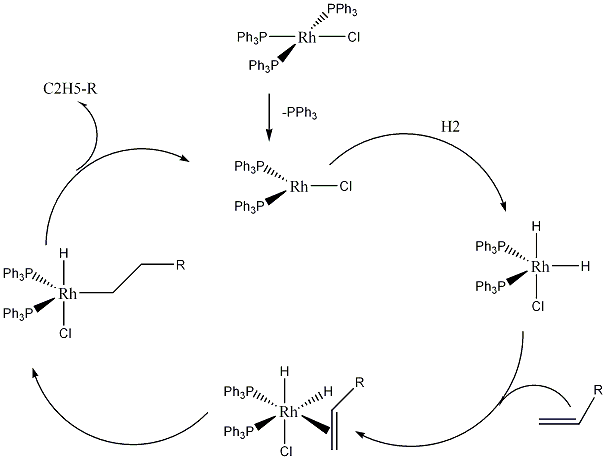
Wilkinson's catalyst catalyzes the hydrogenation of alkenes. The reaction involves the initial dissociation of one or two triphenylphosphine ligands to give 14- or 12-electron complexes, respectively. It is then followed by oxidative addition of H2 to the metal. Subsequent π-complexation of alkene, intramolecular hydride transfer, and reductive elimination then results in extrusion of the alkane product.
Other applications of Wilkinson’s catalyst include: catalytic hydroboration of alkenes and the selective 1,4-reduction of α, β-unsaturated carbonyl compounds in concert with triethylsilane.[6]
Ilustration of Catalytic Chemistry [7]
"http://www.chem.ox.ac.uk/shockwave/hydrogenation.html"
Comparison
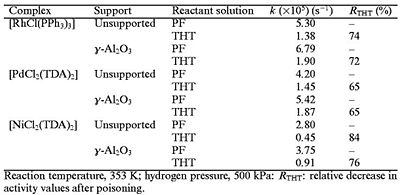
As shown in the tabulated data, Wilkinson catalyst is more active, resulting in faster reactions. Another advantage of Wilkinsons' Catalyst is that it can either be homogeneous or heterogeneous.
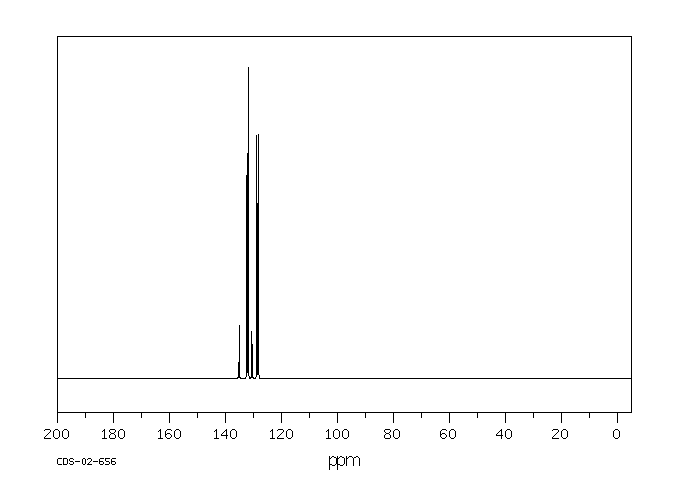
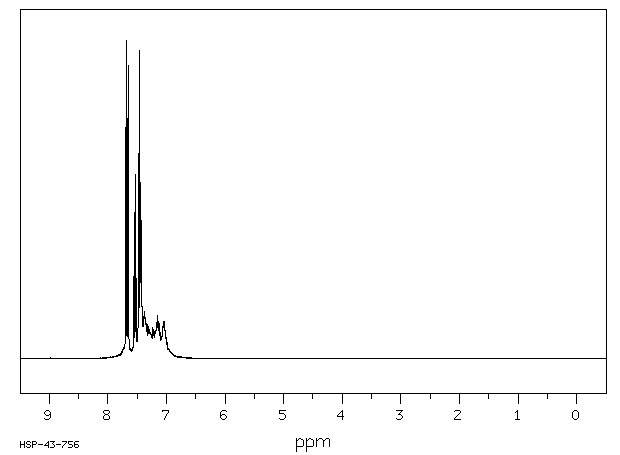
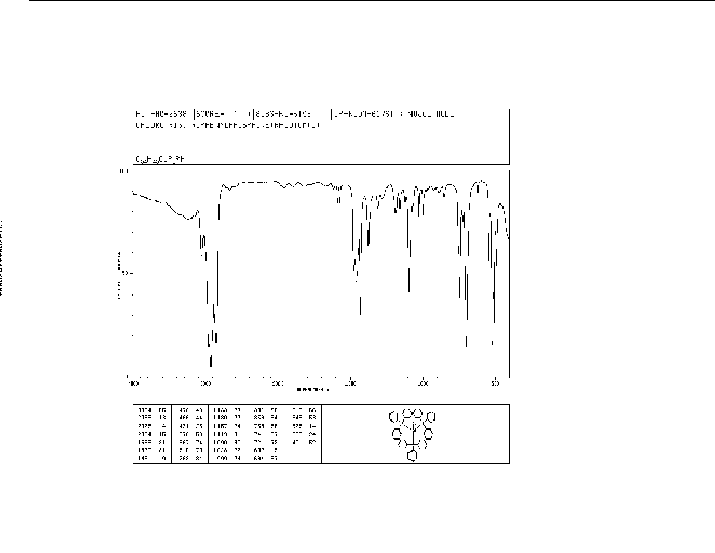
spectrum
MS [1]
Reaction scheme
Applications of Wilkinson's Catalyst
One of the major uses of wilkinson's catalyst is it's use in the hydrogentation of alkenes.
References
- http://en.wikipedia.org/wiki/Wilkinson%27s_catalyst
- http://www.3dchem.com/molecules.asp?ID=35
- http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/205036
- Osborn, J. A.; Jardine, F. H.; Young, J. F.; Wilkinson, G.; Journal of the Chemical Society A, 1966, pp. 1711 - 1732, DOI: 10.1039/J19660001711
- direktinfo.com/cmu/material/Presentations/Wilkinson%20Catalysis.ppt
- http://nobelprize.org/nobel_prizes/chemistry/laureates/1973/wilkinson-autobio.html
- www.chemie.de/lexikon/e/Wilkinson's_catalyst