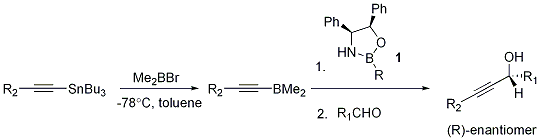It07:oxazaborolidines
Oxazaborolidines
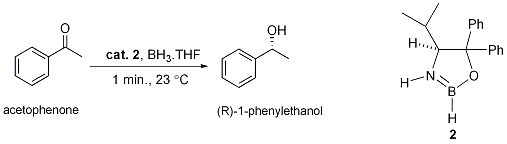
Chiral oxazaborolidines for the enantioselective reduction of ketones were first described by E. J. Corey et al.[1] in 1987. The group found that the reaction of catalytic amounts of oxazaborolidine 2 (0.1 or 0.025 equiv.) with acetophenone in the presence of 1.2 equiv. BH3.THF led to complete reduction of acetophenone to (R)-1-phenylethanol with 94.7% enantiomeric excess (ee) in less than one minute at 23°C.
This was a significant improvement of the methods available for reduction at that time and oxazaborolidines in general have since been developed to catalyze other reactions such as Diels-Alder reactions.
This particular kind of chiral catalyst has been named after the three chemists Corey, Bakshi and Shibata, who published the original article, and is now commonly referred to as CBS catalyst.
Mechanism of the enantioselective reduction of ketones
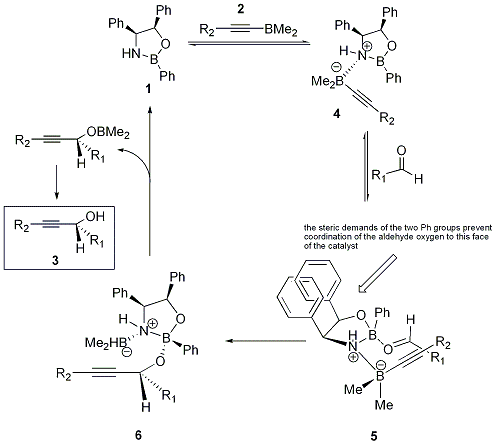
Complex 4 has the necessary structural features to act as an enantioselective reducing agent for carbonyl groups. The ketone oxygen coordinates to the electrophilic ring boron, and this happens in an anti fashion to the larger of the two carbonyl substituents. It is this step which determines the face to which the hydride is eventually delivered.
The molecule is then lost from the catalyst by reaction with BH3. Aqueous work-up gives the desired enantiomer of the alcohol and boric acid.
This method of reduction is so valuable because it is very fast – most reactions are over after one minute of mixing the reagents. Also, the catalyst (or rather the prolinol ligand) is easily recovered in the work-up procedure, which reduces waste and makes the process economical and thus suitable for large scale synthesis.
2 is made by the reaction of (S)-2-amino-3-methyl-1,1-diphenylbutan-1-ol with borane. The reaction is carried out at 0°C for several hours as molecular hydrogen gas is evolved. The electrophilic boron forms a bridge between the Lewis basic nitrogen and oxygen atoms.
Alkynylation of Aldehydes
A further application of chiral oxazaborolidine catalysts lies in the enantioselective nucleophilic alkynylation of carbonyl compounds.[2] Until 13 years ago, the methods available had only moderate enantioselectivities.
The oxazaborolidine catalyzed reaction of alkynylboranes with aldehydes is very similar in mechanism to that of the enantioselective reduction of ketones described above.
The general scheme of this type of reaction is outlined below. The alkynylation reagent 2 is made by reacting bromodimethylborane with the correct alkynyl stannane. When 2 is added to aldehyde in the presence of a catalytic amount of oxazaborolidine 1, the (R) isomer of the desired alcohol is obtained in high yield ee ≈ 90%. The chiral catalyst can be easily recovered as its hydrochloride salt.
One face of the oxazaborolidine ring is sterically shielded by the phenyl groups, so the dimethylborane carrying the alkynyl substituent coordinates to the nitrogen at the other face. The aldehyde coordinates to the ring boron on the same face of the ring (i.e. in syn fashion). In analogy to the ketone reduction, the aldehyde oxygen coordinates to boron so that the large alkyl group R1 is anti to the B-O bond, as this avoids an unfavourable steric interaction between the phenyl group on boron and R1.
Thus, the reagents are now attached to the oxazaborolidine in a way that permits delivery of the alkynyl moiety to one particular face of the aldehyde only; this feature makes the reaction very similar to the hydride delivery in the enantioselective reduction of ketones.
|
Oxazaborolidineplusalkynyl |
The jmol model above shows the catalyst with both the aldehyde and alkynyl moiety attached to it. The pink atoms represent the R groups on the ring boron and the aldehyde, respectively. On boron, this is generally a phenyl group. Rotation about the N-B bond is possible, and the most favourable arrangement is that in which the alkynyl moiety is very close to the aldehyde so that delivery is easily possible.
It is important that there is a phenyl group on B and a hydrogen on N. If Ph is replaced by Bu, then the reaction requires a stoichiometric amount of oxazaborolidine. If the H on nitrogen is replaced by an alkyl substituent, no reaction takes place. This is probably due to the steric bulk of alkynyldimethylborane – the borane species cannot coordinate easily to the nitrogen if there is a bulky substituent on it. Overall, the reaction is quite sensitive to steric effects, and it helps when the R1 group of the aldehyde is not sterically demanding.
Asymmetric Diels-Alder Reactions
Oxazaborolidine type catalysts have also been employed in the highly enantioselective catalysis of Diels-Alder reactions. For this, tryptophan derived oxazaborolidines such as 3 have been developed and have been shown to be highly efficient even with rather complex diene components. This is exemplified by the first catalytic enantioselective total syntheses of cassiol , 1, and gibberellic acid by Corey et al.[3]
The reaction becomes susceptible to the action of the catalyst since the dienophile is a 2-substituted acrolein which naturally has a carbonyl group. This can coordinate to the ring boron of the oxazaborolidine and thus sets the stage for high selectivity of the exo over the endo product or vice versa.
The key step in the synthesis of cassiol is the Diels-Alder reaction of 2 and 2-methylacrolein, catalyzed by 25 mol % of 3. The product 4 was obtained in 83% yield with 97% ee.