It07:name of project
Vitamin C14:26, 18 October 2007 (BST)Zl406
| Vitamin C | |||||
|---|---|---|---|---|---|
 | |||||
 | |||||
|
| |||||
| General | |||||
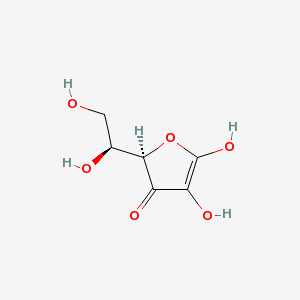
| Systematic name | (2R)-2-[(1S)-1,2-dihydroxyethyl]-4,5-dihydroxyfuran-3-one | ||||
| Other names | Ascorbic acid | ||||
| Molecular formula | C6H8O6 | ||||
| SMILES | O=C1[C@]([C@@H](O)CO)([H])OC(O)=C1O | ||||
| Molar mass | 176.12412 g/mol | ||||
| ATC | A11G | ||||
| CAS number | [50-81-7] | ||||
| PUB CHEM | [644104] | ||||
| synonyms | [L-ascorbate] | ||||
| Properties | |||||
| Melting point | 190-192 (374-378°F) decomposes | ||||
| Pharmacokinetic data | |||||
| Bioavailability | [rapid & complete] | ||||
| protein binding | [negligible] | ||||
| Half life | [16 days, 3.4 hours in people who have excess level of vitamin c] | ||||
| Excretion | [renal] | ||||
| Therapeutic considerations | |||||
| Pregnancy cat. | [A] | ||||
| legal status | [General public availability] | ||||
| Routes | [Orals] | ||||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |||||
Vitamin c is also called L-ascorbate, it is known as the ascorbic acid, which is the only vitamin is water-soluble, unlike other vitamins, most of them are fat-soluble. Unlike most mammals, humans do not have the ability to make their own vitamin C. so we have to obtain vitamin C daily in our diet. It is very essetial to all living animals to form the metabolic reactions and as for all living organisms, plants in this case, human is the only excption. Vitamin C deficiency accelerates aging, this is because it act as an antioxidant in the body, so it protect the living body from over oxdative stress. Finally it also involves in sereval vital enzymatic reactions. But the daily requirement of vitamin c is still under debating since the human body dose not store any vitamin c. Vitamin C is a water-soluble vitamin that is essential for all humans and a few other mammals that lack the ability to biosynthesize the compound from glucose because they lack the enzyme gulono-
Vitamin C functions physiologically, it is a water-soluble antioxidan by its virtue of its high reducing power. It is a essential co-factor for enzymes working involved in the biosynthesis of collagen, carnitine, and neurotransmitters in vitro, and a variety of reactive osygen species and reactive nitrogen species in aqueous envioments can be quenched. Evidence are shown for in vivo antioxidant functions of ascorbate include the scavenging of reactive oxidants in activated leukocytes, lung, and gastric mucosa, and diminished lipid peroxidation as measured by urinary isoprostane excretion. Water-soluble vitamin C also protects fat-soluble vitamins (like A and E) and fatty acids from oxidizing in the body.

Beauty uses
it is believed that overdoes of vitamin c intake daily could whitening the skin and regenerating the skin.
Whitening effect
in the easten world, woman are usually taking overdoes of vitamin c to whitening the skin, also there are serveral ways to intake vitamin c, including oral, liquid , medical .
Regenerating skin
vitamin c can regenerating skin is still unsure, but a lot of woman are taking overdoes vitamin c to reduce the oldening of the skin, this is still because it is a good antioxidant.
Daily requirement
To provide a good antioxidant protection, a Recommended Dietary Allowance (RDA) is set based on the vitamin c intake to maintain near-maximal neutrophil concentration with minimal urinary excretion of ascorbate, which is 90 mg/day for adult men and 75 mg/day for adult women.smoking increases oxidative stress and it also increases metabolic turnover of vitamin C, the requirement for smokers is increased by an average of 35 mg/day. overall estimates of median dietary intakes of vitamin C for adults are 102 mg/day in the US and 72 mg/day in Canada. The Tolerable Upper Intake Level (UL) for adults is set to be at 2 g/day; the adverse effects of overdoes vitamin c upon which the UL is based on the osmotic diarrhea and gastrointestinal disturbances.
Toxicity
it is still unsure that if an large amount of vitamin c intake could result the emitting of toxic in the body, but if eating a vitamin c rich diet, it is not nessesary to intake vitamin c pills.
Vitamin C in diet
RDA-Louis Warschaw Prostate Cancer Center Recommendations
- 60 mg is the daily RDA (recommended dietary allowance) per day. Taking up to 250 mg per day is still acceptable, as long as it comes from your five or more servings of fruits and vegetables.
- a vitamin C supplement have to be taken under care. eating a diet rich in fruits and vegetables is enough amount of vitamin c for daily requirments.
- Consult a physician if undergoing radiation or chemotherapy. eg, it is possible that overdose of vitamin C can interfere with the chemotherapy effects.
Relationships with adiposity
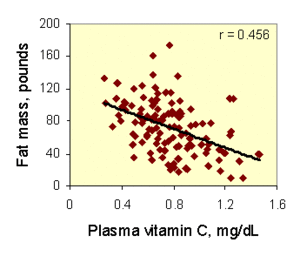
plasma vitamin C concentration is inversely related to fatness, it also inversely related to waist circumference in healthy adults [Journal of Nutrition 2007, In Press]: that is, the more vitamin C stored in plasma, the less body fat in a healthy adults. This may be partially explained by vitamin C’s role, it acts as a cofactor for carnitine synthesis (a molecule which is needed to oxidize body fat).
Fruit and Vegetables Compared and Ranked by Vitamin C
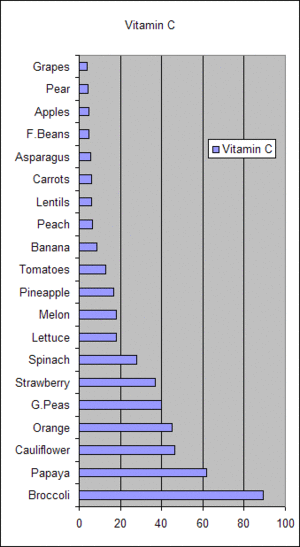
| Friuts and vegetables contains Vitamin C | Value in mg per 100 grams of edible portion of raw item | |
|---|---|---|
| Broccoli | 89.2 | |
| Papaya | 61.8 | |
| Cauliflower | 46.4 | |
| Orange | 45 | |
| G.Peas | 40 | |
| Strawberry | 37 | |
| Spinach | 28.1 | |
| Lettuce | 18 | |
| Melon | 18 | |
| Pinapple | 16.9 | |
| Tomatos | 12.7 | |
| Banana | 8.7 | |
| Peach | 6.6 | |
| Lentils | 6.2 | |
| Carrots | 5.9 | |
| Asparagus | 5.6 | |
| F.Beas | 4.6 | |
| Apple | 4.6 | |
| Pear | 4.2 | |
| Graps | 4 |
References
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5785#Properties http://images.google.co.uk/imgres?imgurl=http://medicineworld.org/images/blogs/7-2007/vitamin-c-vitamin-c-foods-8570.jpg&imgrefurl=http://medicineworld.org/news/news-archives/health-news/Aug-10-2007.html&h=320&w=400&sz=24&hl=en&start=10&um=1&tbnid=9lnrXdh5YrukxM:&tbnh=99&tbnw=124&prev=/images%3Fq%3Dvitamin%2Bc%2Bimage%26svnum%3D10%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-GB:official%26hs%3DpPQ%26sa%3DX http://images.google.co.uk/imgres?imgurl=http://www.poly.asu.edu/ecollege/nutrition/research/Images/johnston/vitaminC.gif&imgrefurl=http://www.poly.asu.edu/ecollege/nutrition/research/vitaminc.html&h=294&w=345&sz=4&hl=en&start=8&um=1&tbnid=0_V3_6GXPsI4XM:&tbnh=102&tbnw=120&prev=/images%3Fq%3Dvitamin%2Bc%2Bimage%26svnum%3D10%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-GB:official%26hs%3DpPQ%26sa%3DX http://images.google.co.uk/imgres?imgurl=http://www.health-garden.com/vitamin-c.gif&imgrefurl=http://www.health-garden.com/vitamin-c-compared.html&h=618&w=339&sz=11&hl=en&start=14&um=1&tbnid=4taBsR-U1umpGM:&tbnh=136&tbnw=75&prev=/images%3Fq%3Dvitamin%2Bc%2Bimage%26svnum%3D10%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-GB:official%26hs%3DpPQ%26sa%3DX
- 1. J. L. Chavez-Servin, A. I. Castellote, M. Rivero and M. C. Lopez-Sabater, Food Chemistry, 2008, 107, 1187-1197.
- 2. M. da Silva Pinto, F. M. Lajolo and M. I. Genovese, Food Chemistry, 2008, 107, 1629-1635.
- 3. S. Landon, Food Australia, 2007, 59, 533-538.
- 4. Application: CNCN Pat., 1004-6616101077352, 2007.
- 5. L. R. Moser and A. B. Ordman, Age (Dordrecht, Netherlands), 2006, 28, 77-84.
- 6. Application: CNCN Pat., 1008-5953101077372, 2007.
