It07:linalool
Linalool
| Template:Linalool | |
|---|---|

| |
| Synonyms | 3,7-Dimethyl-1,6-octadien-3-ol Linalyl alcohol allo-Ocimenol 2,6-Dimethyl-2,7-octadien-6-ol Licareol (l-Linalool) Coriandrol (d-Linalool) |
| Molecular formula | C10H17OH |
| SMILES | C/C(C)=C/CCC(C=C)(O[H])C |
| Physical State&Appearance | colourless liquid, with characteristic odour |
| Molar mass | 154.2 g/mol |
| Properties | |
| Relative Density | 0.858 – 0.868 g/cm3 |
| Melting point | < 20 °C |
| Boiling Point | 198 – 200 °C |
| Vapour Pressure | 21pa (at 25 °C) |
| Water Solubility | 854 mg/l (23.5 °C) – 1589 mg/l (25 °C) |
| Partition coefficient n-octanol/water(log value) | log POW = 2.97 (23.5 °C) |
| Henry’s law constant | 1.9 · 10-5 atm·m3/mol |
| BCF Bioconcentration Factor | 28 (QSAR estimate) |
| Surface Tension | 20.969 mN/m (20 °C) |
| Flash Point | 75 °C |
| Auto-igniton Temperature | 235 °C |
| Hazards | |
| Human Health Hazards | (Moderate) Irritant of eyes and skin |
| Chemical Dangers | the substance decomposes on heating producing acrid smoke and irritating fumes. Reacts with strong oxidants. |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Introduction
Linalool is a colourless, fragrant liquid at room temperature, with molecular formula C10H18O. It’s a molecule (terpenoid alcohol) found in nature which can be distilled from the oils of bergamot, rosewood and other trees and plants by biosynthesis. Pure linalool can also be created by chemical synthesis. Tracing back, the first synthetic linalool was produced in the 1920s. The synthetic linalool is a so-called “nature identical” as molecularly, synthetic and natural linalool exactly the same.
3D Structure
Cyclopentasiloxane |
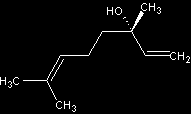
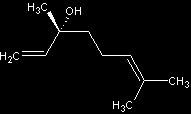
Stereoisomers of Linalool
- (R)-(-)-linalool - floral, woody lavender note
- Odor Threshold = 9-11 ppb in air (Christoph); 0.8 ppb (Padrayuttawat et. al.)
- (S)-(+)-linalool - sweet, floral; odor reminiscent of petitgrain and lavender
- Odor Threshold = 35-40 ppb in air (Christoph); 7.4 ppb (Padrayuttawat et. al.)
Uses of Linalool
Since linalool has flavouring and fragrant properties, one of its most significant uses is in perfume manufacture. It can be found as one of the essential ingredients in most perfumes. Linalool also applies widely in many other fields. It is added to processed beverage and food, cosmetics and soaps as well as to waxes and household detergents. What is more, its application also can be found in the Vitamin E synthesis and for stored-food pest control.
Synthesis of Linalool
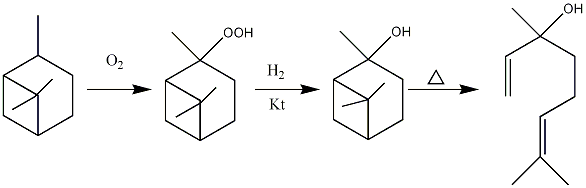
Linalool has stereoisomerism properties, existing as (S)-linalool and (R)-linalool corresponding to the stereo centre hydroxylated third carbon. One of the common ways to make linalool is from alpha- or beta-pinene by chemical synthesis. alpha-Pinene is hydrated selectively turning to cis-pinane which can be subsequently oxidised to trans/cis (25%/75%) pipane hydroperoxide. Under specific conditions this intermediate products can turn into pinanols and finally pyrolyse to the respective linalool.
Storage
Liquid linalool should be kept in a dry and light blocked glass container with tight sealing. The bottle which contains linalool should be kept in a cool and dry place.
Reference
1.http://www.ilo.int/public/english/protection/safework/cis/products/icsc/dtasht/_icsc09/icsc0912.pdf
2.http://www.inchem.org/documents/sids/sids/78706.pdf
3.http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc09/icsc0912.htm
4.http://www.leffingwell.com/chirality/linalool.htm
5.http://www.umich.edu/~chemh215/CHEM216/HonorsCup/hcproposal/251__A.pdf
6.http://chemse.oxfordjournals.org/cgi/content/full/29/3/253