It07:knots
| Example of a Molecular Trefoil Knot[1] | ||||
|---|---|---|---|---|
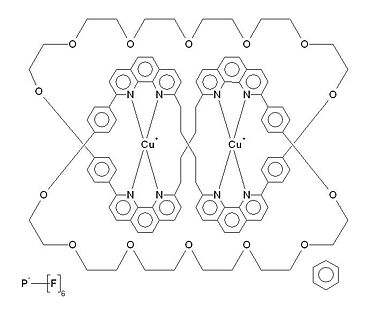
| ||||
| General | ||||
| Systematic name | (9,12,15,18,21,24,27,36,39,42,45,48,51,54-Tetradecaoxa-1,8,28,35(1,4)-tetrabenzena-2,7,29,34(2,9)-tetrakis(1,10-phenanthrolina)cyclotetrapentaphane)-di-copper(i)-trefoil knot bis(hexafluorophosphate) benzene solvate | |||
| Molecular Formula | C104H104Cu2N8O142+, 2(F6P)-, C6H6 | |||
| Colour | Dark Red | |||
| Molar mass | 2203.017 gmol-1 | |||
Introduction
A molecular knot occurs when a molecule is intertwined in such a way to produce a nanoscale equivalent to a macroscopic knot, such as a trefoil knot - an example of a synthetic molecular knot shown right. There are also examples of naturally occurring molecular knot structures, as well as synthetic ones, such as DNA and some proteins. A trivial name is sometimes also used to describe molecular knots – ‘knotanes’ (a term used by Fritz Vögtle et al in Angewandte Chemie International Edition in 2000). This term arose from the similarity to rotaxane, which consists of a macrocyclic ring attached to a chain molecule that is larger on both ends to prevent the ring becoming detached, and catenane, which consists of two or more interlocked macrocyclic rings.
Syntheses
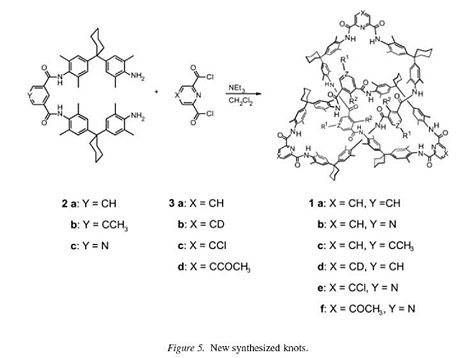
For a long time, synthesis of these knotted molecules, such as the molecule in the example shown right, was considered to be just a theoretical procedure that was not physically possible. However, the emergence of the supramolecular template method for synthesis has changed this view and enabled the production of such molecule.[2] Several topologically chiral knotted molecules made with amide bonds were synthesised by J. Recker and F. Vögtle[3] via this method in their paper ‘Amide-Based Molecular Knots’ published in 2003. It was possible to achieve a yield for these of around 20%. The knots were made from the following compounds: an isophthalic acid dichloride, a diphenylmethane derived diamine and a pyridine-2,6-dicarboxylic acid dichloride. An equation for the formations is shown in the picture:
With regards to the mechanism for the formation of the knotted molecules, no external template was used, instead ‘all six educt molecules are reacting in an internal templating reaction.’ X-Ray analysis and chromatographically separatable enantiomers were used to prove that this trefoil molecular knot based on amide bonds was indeed formed.
Uses
Now, because of the new technology discussed above, knotted molecules can be synthesised for use in molecular machines and nanoscale sensors/switches. Although giant strides have been made with regards to the synthesis of knotted molecules and the recognition that they are useful building blocks in the development of molecular machines, there are not yet any molecular devices that are made from knotted molecules. Some reasons for this are due to difficulties in ‘preparative-scale synthesis, purification and especially derivitization of the molecular knots.’ A paper published in 2003 by F. Vögtle et al states that conformational properties of these knotted molecules are of major importance in the formation of molecular switches due to the fact that they depend on controlled conformational transitions.[4] At the present time, the ‘solvent- and temperature-dependant conformational equilibrium’ for knotted molecules is being investigated in order to try to find a solution to the problems stated, and eventually enable molecular devices to be constructed from these knotted molecules in question.
Future Development Possibilities
Using the supramolecular template method for synthesis of knotanes, there are many possibilities for further knot structures/other structures with ‘twisted architectures’ to be created that have not to date been made, for example the theoretical structure shown in the picture[5] - a heptafoil knot with seven trefoil knots attached:

References
- ↑ C.O.Dietrich-Buchecker, J.Guilhem, C.Pascard, J-P.Sauvage, Angew.Chem., Int.Ed. 1990, 29, 1154
- ↑ O. Lukin, W. M. Muller,U. Muller, A. Kaufmann, C. Schmidt, Chem. Eur. J. 2003, 9, 3507-3517
- ↑ J. Recker and F. Vögtle, Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2001, 41, 3–5
- ↑ O. Lukin, W. M. Muller,U. Muller, A. Kaufmann, C. Schmidt, Chem. Eur. J. 2003, 9, 3507-3517
- ↑ O. Lukin and F. Vögtle, Angew.Chem., Int.Ed 2005, 45/4