It07:Warfarin
| Warfarin | |
|---|---|
| |
| IUPAC Systematic name | |
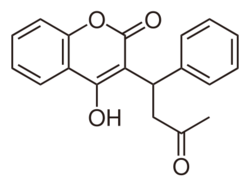
| (RS)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2H-chromen-2-one | |
| Other name | |
| Coumadin, Jantoven, Marevan, Waran&btnG=Google+Search&meta= Warfarin, Coumadin, Jantoven, Marevan, Waran | |
| Indentifiers | |
| ATC Code | {{{ATC_Code}}} |
| CAS number | {{{CASNo}}} |
| PubChem (CID) | {{{PubChem}}} |
| SMILES | O=C2C(C(CC(C)=O)C3=CC=CC=C3)=C(O)C1=CC=CC=C1O2 |
| Chemical Data | |
| Molecular formula | C19H16O4 |
| Molar mass | 308.33 g/mol -1 g/mol |
| Pharmacokinetic Data | |
| Bioavailability | {{{Bioavailability}}} |
| Protein Binding | {{{Protein_binding}}} |
| Metabolism | {{{Metabolism}}} |
| Half life | {{{Half_life}}} |
| Excretion | {{{Excretion}}} |
| Therapeutic considerations | |
| Pregnancy cat. | {{{Pregnancy_cat}}} |
| Legal status | {{{Legal_status}}} |
| Routes | {{{Routes}}} |
Basic Information
Warfarin is a commonly prescribed pharmaceutical molecule principally used as an anti-coagulant, hence is used to thin the blood and prevent clotting from occurring. Other common anti-coagulants include Heparin and all work by decreasing the ability of the blood to clot.Warfarin was first synthesised as an effective rodenticide(rat poison) however as more potent and effective molecules were found use for this purpose was ceased. Warfarin, and the other rodenticides are in fact all part of the same related family of molecules, coumarins, which all work in similar ways to prevent blood clotting.
The coumarin class of drugs are all synthetically manufactured molecules based on coumarin, a natural chemical found plants, such as sweet clover as discussed as well as woodruff[1] (Galium odoratum, Rubiaceae). In addition lower levels of coumarin are found in licorice, lavender as well as other species. Coumarins work to limit coagulation by changing the rate and efficiency of the recycling of Vitamin K. Warfarin inhibits the action of vitamin K reductase, hence preventing the recycling of oxidated vitamin K. This results in a lack of oxidised vitamin K and thus a limit in the amount of clotting possible for the blood. Due to their antagonistic action the coumarins are known by the pseudonym of as vitamin K antagonists.
Jmol 3D structural views of molecule:
History
The synthesis of warfarin or more specifically the class of drugs now commonly referred to as coumarins began in the early 1940’s by a process of experimentation. It had been observed that ‘sweet clover’ had a toxic effect and experimentation by Karl Link and his student Harold Campbell at the University of Wisconsin led to the discovery that this toxicity was due to the fungus aspergillis which produced a toxic agent bishydroxycoumarin[2].
Further work then occurred to investigate and improve the potency and stability of this molecule and after further development it was noticed that this molecule had an antagonistic effect on the action of vitamin K, important in blood clotting, thus was an effective anticoagulant. Research continued, culminating in the application for a patent on warfarin (so called after the Wisconsin Alumni Research Foundation).
Mechanism of Action
Warfarin works as a vitamin K inhibitor. Vitamin K provides a number of the factors required for clotting, hence warfarin is successful in preventing blood clotting by stopping these factors working. The clotting factors in question require carboxylation of their acid residues in order to be able to bind correctly and hence initiate clotting.
Warfarin works by inhibition of epoxide reductase which is responsible for recycling vitamin K to keep concentration high. As this is inhibited by warfarin the amount og vitamin K is not as readily regulated by the action of the enzyme. This results in a decreased carboxylation of required clotting factors which has the immediate effect of preventing the clotting factors from being able to bind to the surface of blood vessels. Unable to bind to blood vessels the clotting, or more precisely coagulation, of blood is not possible and hence a decrease in clotting results.
Warfarin has an initial effect of increasing coagulation, hence having the reverse role to that desired. This is due to the initial effect of the promotion of another clotting factor which therefore causes increased clotting to occur.
Synthesis
Warfarin is readily synthesised from 4-hydroxycoumarin and benzalacetone. This is done by a Michael condensation reaction in water resulting in the formulation of the desired molecule.
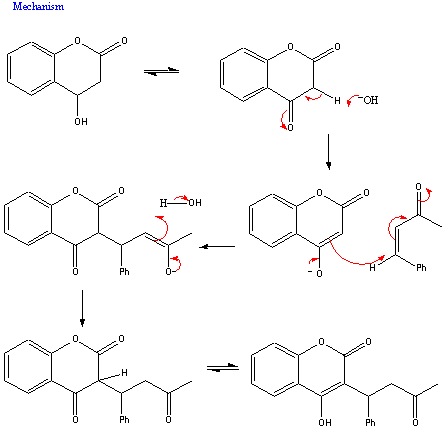 [3]
[3]
Pharmacology
Warfarin is one of the major anticoagulant molecules on the market however it has the initial effect of doing the reverse of its therapeutic effect. A further problem is the slow acting nature of the molecule. These problems are countered by coupling the administration of warfarin with administration of heparin. Warfarin is given orally in dosages of 1mg, 3mg and 5mg and combinations of these. These are distinguished in the UK by the different colours of the different tablets with 1mg being brown, 3mg being blue and 5mg being pink.
When a clot is noticed heparin is first injected and then warfarin is gradually dosed. This means that clotting is prevented throughout by first the injection of heparin and secondly the oral administration of wafarin.
Drug-Drug Interactions
Warfarin interacts with a number of other molecules, food stuffs as well as many herbs and spices. As a result it is very important to monitor patients regularly to ensure adequate and not excessive doses of warfarin are being given. Herbs which increase blood pressure and bleeding have an opposite effect to warfarin. Both garlic and ginger## cause an antagonistic effect by increasing bleeding, as a result levels of these consumed by patients should be limited.
