It07:Vitamin E
Vitamin E
Vitamin E comprises of a group of biological molecules that are all derivatives of either Tocopherol or Tocotrienol. The most bioactive naturally occuring forms of vitamin E is α-Tocopherol and it is found in green vegtables, grains as well as palm, olive and safflower oils. Pure crystaline α-Tocopherol appears as transparent needles with a melting point 275.65-276.65K.
Vitamin E has long been marketed as a health supplement as it acts as a powerful antioxidant. The received wisdom being that it helps to reduce the chance of tissue and cellular damage by neutralizing free radicals in the body. The idea that antioxidant supplements have a positive effect on the body has, in recent years, been widely debunked but a series of studies, and now the general consensus in the scientific community is that antioxidants can only give health benefits while still in situ; in fresh fruits, vegetables and teas.[1]
Vitamin E also plays a very important part in medical uses. It helps to keep the circulatory system healthy by preventing the blood clotting. Some survey also indicates that vitamin E decreases the symptoms of some kinds of breast disease. Some studies showed that taking large amount of vitamin E can decrease the risk of Coronary Artery Disease (CAD). But these studies have still not been verified with large scale clinical trials, so taking large doses of vitamin E is still not recommended.
Stuctures of Vitamin E
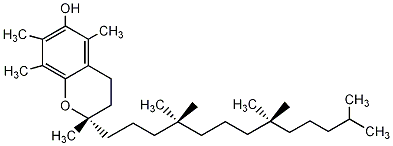

Solubility of vitamin E
Vitamin E is soluble in fat issues while some other vitamins are soluble in water. Although vitamin E has been proven to have lots of advantages for human body, over amount of vitamin E can store in livers and cause health problem. Taking too many fat-soluble vitamins, i.e. vitamin E, can lead to toxicity. Normal health diet should apply right amount of vitamins.
Storage
Vitamin E should be stored in a dry place at room temperature.
Deficiency
Children and adults who are unable to absorb and utilise vitamin E can develop a characteristic and progressive neurological syndrome, which affects the central and peripheral nervous system, skeletal muscle and retina. The requirements for vitamin E is determined largely by the polyunsaturated fatty acid (PUFA) content of tissues, which in turn reflects the PUFA content of the diet. Owing to the widely differing requirements based on the PUFA intake, it is generally considered more realistic to give ranges of acceptable intake rather than a fixed level. It is generally recommended that that daily intakes of 4 mg and 3 mg of α-tocopherol equivalents could be adequate for men and women respectively (COMA, 1991). If the lower intakes are maintained over prolonged periods, there is an increased potential for deficiency. [2]
| α-Tocopherol | ||||
|---|---|---|---|---|
| It07:Vitamin E | ||||
| General | ||||
| Systematic name | (2R)-3,4-Dihydro-2,-5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-2H-1-benzopyran-6-ol | |||
| Other names | {{{OtherNames}}} | |||
| Molecular formula | C29H50O2 | |||
| SMILES | CC(C)CCC[C@@H](C)CCC[C@@H](C)CCC
[C@]1(C)CCc2c(C)c(O)c(C)c(C)c2O1 | |||
| Molar mass | 430.70 g/mol | |||
| Appearance | Transparent needles | |||
| CAS number | {{{CASNo}}} | |||
| Properties | ||||
| Density & phase | {{{Density}}} g/cm³ | |||
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) | |||
| Melting point | {{{Mp}}} 275.65-276.65K | |||
| Boiling point | {{{Bp}}} K | |||
| Acidity (pKa) | {{{pKa}}} | |||
| Basicity (pKb) | {{{pKb}}} | |||
| Chiral rotation [α]D | {{{Rotation}}}° | |||
| Viscosity | {{{Viscosity}}} cP at 25°C | |||
| Structure | ||||
| Molecular shape | {{{Mol_Shape}}} | |||
| Coordination geometry |
{{{Coordination}}} | |||
| Crystal structure | {{{Crystal_Structure}}} | |||
| Dipole moment | {{{DM}}} D | |||
| Hazards | ||||
| MSDS | External MSDS | |||
| Main hazards | {{{Hazards}}} | |||
| NFPA 704 | {{{NFPA}}} | |||
| Flash point | {{{Fp}}}°C | |||
| R/S statement | R: {{{R-S}}} S: ? | |||
| RTECS number | {{{RTECS}}} | |||
| Supplementary data page | ||||
| Structure and properties |
n, εr, etc. | |||
| Thermodynamic data |
Phase behaviour Solid, liquid, gas | |||
| Spectral data | UV, IR, NMR, MS | |||
| Related compounds | ||||
| Other anions | {{{Other_anion}}} | |||
| Other cations | {{{Ohter_cation}}} | |||
| Related compounds | {{{Relative_Compounds}}} | |||
| 3-D Structure | ||||
| 3D |
| |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Sources
1. M. J. O'Neil, The Merck Index; Thirteenth Edition, 2001, p1693 2. http://news.bbc.co.uk/1/hi/health/6399773.stm - Antioxidants incresing cancer risk
