It07:Vioxx
Appearance
| Vioxx | |
|---|---|
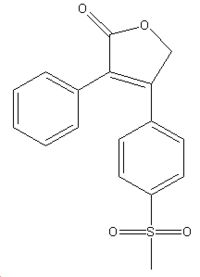
| |
| IUPAC Name | |
| 4-(4-methylsulfonylphenyl)-3-phenyl-5H-furan-2-one | |
| Other name | |
| Rofecoxib | |
| Identifiers | |
| ATC Code | {{{ATC_Code}}} |
| CAS number | 162011-90-7 |
| PubChem (CID) | 5090 |
| SMILES | CS(=O)(=O)C1=CC=C(C=C1)C2=C(C(=O)OC2)C3=CC=CC=C3 |
| Chemical Data | |
| Molecular formula | C17H14O4S |
| Molar mass | 314.35566 g/mol |
| Physical Data | |
| Melting Point | 204.7°C [1] |
| Pharmacokinetic Data | |
| Bioavailability | 93% |
| Protein Binding | 87% |
| Metabolism | Hepatic |
| Half life | 17 hours |
| Excretion | biliary |
| Therapeutic considerations | |
| Pregnancy cat. | C |
| Legal status | withdrawn |
| Routes | oral |
Introduction
Vioxx is a COX-2 selective nonsteroidal anti-inflammatory drug (NSAID) developed by Merck. It was seen as a breakthrough in pain relief for arthritis sufferers due to its lack of side effects. Merck withdrew the drug in September 2003.
Controversy
On September 30, 2004 Merck voluntarily withdrew Vioxx from the market. This was the result of several studies, but, in particular Mercks own study; APPROVe (Adenomatous Polyp PRevention On Vioxx).
APPROVe
In 2001, Merck began a three year study with the main aim being to test the efficacy of Vioxx as a prevention to rectal polyps. A second, anillary, study was to evaluate the cardiovascular safety of the drug.

3D Rendition of Vioxx
Vioxx |
References
- ↑ Therien, M; Gauthier, J.Y; Leblanc, Y; Leger, S et al; SYNTBF; Synthesis; EN; 12; 2001; 1778 - 1779
