It07:Vanillin
Introduction
Vanillin is responsible for the smell and flavour of vanilla and is naturally found in vanilla pods. Orginating from Mexico where Aztecs cultivated the pods, Spanish explorers brought them to Europe in 1520[4]. Currently the majority of vanillin produced is synthesised. Fortunately vanillin is one of the most easily recognised flavours, requiring one of the lowest parts per million to be recognised by the human sense of taste.
Synthetic
The major use of vanillin is in sweet foods where the ice cream and chocolate industries account for 75% of the market for vanillin. The global demand of vanillin is currently 12000 tonnes per annum[5].

Vanillin contains an aldehyde, ether and phenol group.
An example of how to synthesise vanillin:
Preparation of vanillin with high yield by reaction glyoxylic acid with 2-methoxyphenol with a catalyst, followed by decarboxylation.[6]
Natural
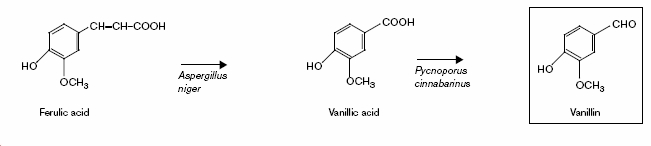
Natural vanillin, whilst only a minor fraction of the vanillin market (since it takes approximately 8 months to cultivate), is in fact 250 times more expensive than the synthetic product. This is due to the presence of other compounds such as p-hydroxy-benzaldehyde which effects the flavour. Hence efforts have been made to determine biosynthetic paths for its production.[7]

Fuleric acid is a common starting point for this synthesis due to its ready availability in bran and barley spent grain. Aspergillus niger contains the enzyme (cinnamoyl esterase) for the first conversion.
Authenticity
Due to the extreme price difference between natural and synthetic vanillin, the authenticity of some vanillin pods are sometimes called into question. Hence a popular technique used to solve this problem is chromotography. Synthetic vanillin has an unuasually large relative amount of vanillin with respect to other benzoic compounds in natural vanillin.[10]
3D Structure
| It07:Vanillin | |
|---|---|
| |
| General | |
| Systematic name | 4-hydroxy-3-methoxy-benzaldehyde |
| Other names | 4-Oxy-3-methoxy-bezaldehyde
4-Hydroxy-3-anisaldehyde Vanillaldehyde |
| Molecular formula | C8H8O3 |
| SMILES | O=CC1=CC(OC)=C(O)C=C1 |
| Molar mass | 152.15 |
| Appearance | White / yellow (very light) solid |
| CAS number | 121-33-5 |
| Properties | |
| Melting point | 354.15-356.15 K[1] |
| Boiling point | 558 K[2] |
| Refractive Index | 1.692[3] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Vanillin |
Unit Cell of cis-Diaqua-bis(vanillinato)-copper(ii)
Vanillin |
Spectra
IR Spectrum[11]
Mass Spectrum[12]
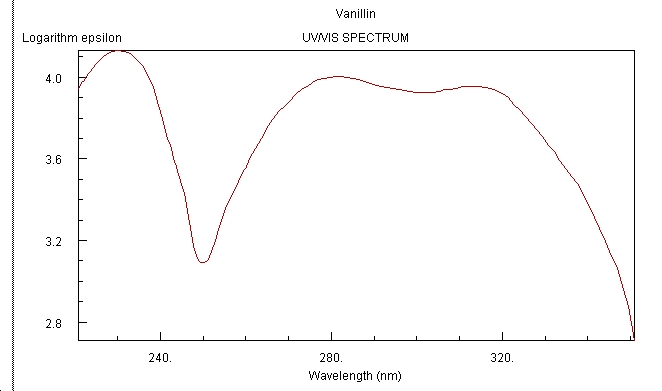
UV/Vis Spectrum[13]
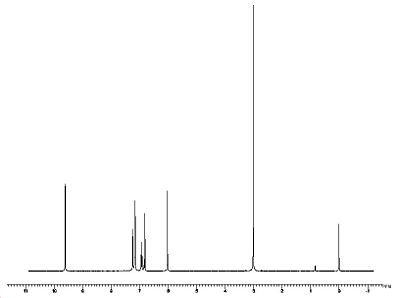
NMR
Spectrum ID: CC-01-H_NMR-598 (high-resolution image)
Spectrometer: BRUKER WM-250
Working Frequency: 250 MHz
Solvent: Benzene-d6 (1076-43-3)
Standard: Tetramethylsilane (75-76-3)
Temperature: 24 °C
[14]
References
- ↑ J. Chem. Soc., Perkin Trans. 2, 1998, 989 - 994, DOI:10.1039/a704633b
- ↑ F. F. Schmitt, Manufacturing chemist, 1955, 26, 541.
- ↑ J. B. J. Wilson, Journal of the Association of Official Agricultural Chemists, 1930, 13, 389.
- ↑ N. J. Walton, M. J. Mayer and A. Narbad, Phytochemistry, 2003, 63, 505-515.DOI:10.1016/S0031-9422(03)00149-3
- ↑ http://www.3dchem.com/molecules.asp?ID=307
- ↑ Publication number: CN1537675 Publication date: 2004-10-20 Inventor: HOU SHULAN (CN); WEI GUOFENG (CN); ZHOU ZIGANG (CN) Applicant: JIHUA GROUP CORP (CN) Classification: - international: B01J27/043; B01J27/051; C07C45/45; C07C47/575; B01J27/04; C07C45/00; C07C47/52; (IPC1-7): B01J27/051; B01J27/043; C07C45/45; C07C47/575 - European: Application number: CN20031033417 20030607 Priority number(s): CN20031033417 20030607
- ↑ J. Thibault, V. Micard, C. Renard, M. Asther, M. Delattre, L. Lesage-Meessen, C. Faulds, P. Kroon, G. Williamson, J. Duarte, J. C. Duarte, B. C. Ceccaldi, M. Tuohy, D. Couteau, S. Van Hulle and H. P. Heldt-Hansen, Lebensmittel-Wissenschaft und-Technologie, 1998, 31, 530-536.DOI:10.1006/fstl.1998.0411
- ↑ http://www.understandingfoodadditives.org/assets/vanillaPod.jpg
- ↑ N. J. Walton, A. Narbad, C. Faulds and G. Williamson, Current Opinion in Biotechnology, 2000, 11, 490-496.DOI:10.1016/S0958-1669(00)00125-7
- ↑ N. J. Walton, M. J. Mayer and A. Narbad, Phytochemistry, 2003, 63, 505-515.DOI:10.1016/S0031-9422(03)00149-3
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C121335&Units=SI&Type=IR-SPEC&Index=2#IR-SPEC
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C121335&Units=SI&Mask=200#Mass-Spec
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C121335&Units=SI&Mask=400#UV-Vis-Spec
- ↑ Spectral data were obtained from Wiley Subscription Services, Inc. (US)