It07:Tropinone
Tropinone
| Tropinone | |
|---|---|
| |
| General | |
| Systematic name | 8-Methyl-8-aza-bicyclo[3.2.1]octan-3-one
|
| Molecular formula | C8H13NO |
| SMILES | |
| Molar mass | 139.2g mol-1 |
| CAS number | 532-24-1 |
| Properties | |
| Density & phase | solid |
| Melting point | 400C |
| Boiling point | 1130C |
| Chiral rotation [α]D | |
| Hazards | |
| MSDS | http://www.sigmaaldrich.com/ |
| Main hazards | Not hazardous according to Directive 67/548/EEC |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Tropinone is an alkaloid that belongs to the atropine group. It was first synthesized by Willstätter[1] in 1896 and subsequently by Sir Robert Robinson[2] in 1917 in a very elegant way. Tropinone is a deep brown, crystalline solid. It was considered a starting point in the synthesis of a number of bases that were important in the medical field such as atropine, homatropine and tropacocaine.
Robinson's Synthesis of Tropinone
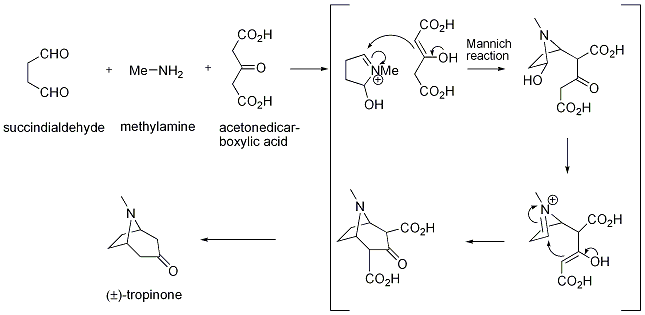
In the original paper that describes the synthesis of tropinone, Robinson described the “imaginary hydrolysis” of tropinone to give succindialdehyde, methylamine and acetone as possible starting materials. This insight eventually made the synthesis so elegant, and this kind of retrosynthetic analysis subsequently became one of the most important concepts in organic chemistry.
The reaction proceeds as follows: Methylamine and succindialdehyde condense to form an iminium ion. This reacts in a Mannich fashion with the enol form of acetonedicarboxylic acid to give a b-amino ketone. Acidic conditions lead to loss of water and generation of another iminium ion, which is again attacked by the enol to close the ring. Acidifying and heating the reaction mixture leads to decarboxylation and thus the tropinone molecule. It was envisaged that this method of making bicyclic bases would be of general utility and applicable to a wide range of target molecules.
Robinson’s synthesis of tropinone illustrated the rapid increase of molecular complexity that accompanies the use of so-called tandem transformations, of which the double Mannich reaction is a nice example.