It07:Thyjone
| It07:Thyjone | |
|---|---|
| |
| Chemical name | (1S-(1-,4-,5-alpha))4-methyl-1-
propan-2-yl-bicyclo[3.1.0]hexan-3-one | |
| Chemical formula | C10H16O |
| Molecular mass | 152.23 g/mol |
| Density | 0.92 g/cm³ |
| Solubility | insoluble (water)
soluble (ethanol) |
| Boiling point | 201 °C |
| CAS number | [546-80-5] (α-thujone)
[471-15-8] (β-thujone) |
| SMILES | C[C@@H]([C@@H](C2)[C@]2
([C@@H](C)C)C1)C1=O (β-thujone) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
overview
Thujone is a terpenoid ketone which exists in two stereo-isomeric forms:
R1 = CH3; R2 = H: (+)-3-thujone and R1 = H; R2 = CH3: (-)-3-thujone
(+)-3-thujone [alpha-thujone] and (-)-3-isothujone[ ß-thujone]. Both of those are natural occurring with a menthol odor. It was first discovered by Dr. Valentin Magnan when studying absinthe. And it has become a myth that absinthe contains a lot of thyjone and it is responsible for the hallucinogenic effects. It was later found that actually only a very small amount of thujone is in absinthe. In many countries the amount of thujone in food products is regulated.

Image taken from http://www1.istockphoto.com/file_thumbview_approve/1185965/2/istockphoto_1185965_two_absinthe_glasses.jpg
3D structure
Thujone |
Biochemical Aspects
Thujone was thought to acts as a inhibitor of acyl-CoA activity in brain synaptosomes. Experiments were done to study the effect of thujone on mouse brain. One involve giving an oral doses of 10-100 mg/kg Thujone to mouse, but no effect on respiratory activity of the cerebral cortex was pretreated. (Pinto-Scognamiglio, 1968).
At the moment there is no scientific evidence on any dose will cause hallucinations.
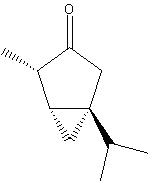
predicted NMR using chemdraw
External links
Thujone.Info — articles on thujone, absinthe, absinthism