It07:Tetrodotoxin
Natural Occurrence
Tetrodotoxin is a naturally occuring neurotoxin, to which there is no known antidote. It is found in a range of poisonous animals such as pufferfish and blue ring octopus. It gets the name tetrodotoxin after Tetraodontiformes, an order of fish many of which carry the toxin. Whilst found in the animals it is actually produced by certain bacteria such as the species Pseudoalteromonas tetraodonis. The bacteria nad the animals have a mutually advantageous symbiotic relationship wheree the animal receives the benfits of the toxin (either for protection or hunting) and the bacteria is provide with food from its host.
| Tetrodotoxin | |
|---|---|
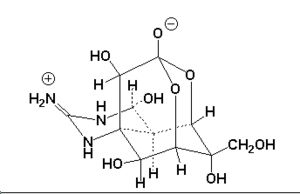 [1] [1]
| |
| General | |
| Systematic name | Octahydro-12-(hydroxymethyl)-2-imino-5,9:7,
10a-dimethano-10aH-[1,3]dioxocino[6,5-d] pyrimidine-4,7,10,11,12-pentol. |
| Other names | Tetrodotoxin |
| Properties | |
| Molecular formula | C11H17N3O8 |
| Molar mass | 319.268 |
| CAS number | 4368-28-9 |
| SMILES | C([C@@]1([C@@H]2[C@@H]3[C@H](N=C(N[C@@]
34C([C@@H]1O[C@@]([C@H]4O)(O2)O)O)N)O)O)O |
| Optical Rotation | -8.64° |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Pentahelicene |
Synthesis
Synthesis of tetrodotoxin is long and complex. It was first achieved in 1972[2]and stereospecific synthesis in 2003 by two different methods[3][4].
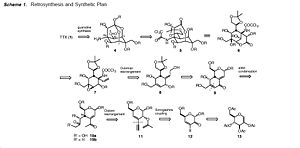
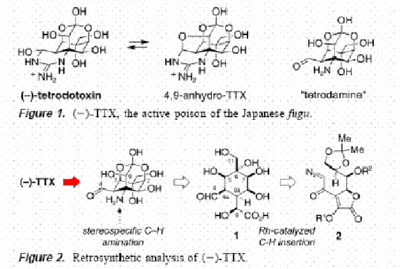
These second generation stereospecific syntheses, are particularly groundbeaking and useful as unlike the original first generation synthesis by Y Kishi and friends (in 1972) which gained the D,L-tetrodotoxin, they form an enatiomerically pure form, like what would be found biologically. Elegance is provided by the A Hinman and J Du Bois synthesis in 2003, which introduces the exciting technique of C-H functionalization to formulate the amine group in the final step.[6]
Tetrodotoxin Poisoning
Tetrodoxin works by mimicking a hydrated sodium ion and entering one of the sodium channels on a nerve cell membrane. Once there it binds to peptide group, making it hard to remove, and blocks the movement of sodium ions through the channel, causing the action potential of the nerve cell to cease. It targets the contractual cells of the muscles causing them to become paralysed, which when occurring in the muscles of the heart can lead to a fatality. A dose of less than 1mg is enough to kill a fully grown adult.
The symptoms of tetrodotoxin poisoning cans be separated into four stages [7]. The first symptom is numbness of lips and tongue. This is followed by paralysis of the extremities and a drop in reflexes. The third stage involves incoordination, respiratory distress and a drop in blood pressure. Stage 4 is mental impairment, a large drop in blood pressure and respiratory paralysis. This is usually followed by death. A quicker progress of symptoms indicates a higher likelihood of death, usually within 4 to 6 hours of ingestion however if the patient is able to survive 24 hours they will most likely go on to make a full recovery [8] There are an estimated 200 cases of tetrodotoxin poisoning each year with a fatality rate of approximately 50%. [9]. Japan has the highest occurrence of tetrodotoxin poisoning, as pufferfish or fugu is considered a traditional delicacy. However the number of deaths is decreasing due to strict licensing on who can prepare the dish [10]
Antidote
Amazingly for such a dangerous substance found in a national cuisine, there is no known antidote to tetrodotoxin. Therefore only basic medical methods can only be applied as treatment such as machine control of respiratory and circulatory systems, as the patient eventually dies from asphyxiation. Ironically there are small non-hazardous quantities of tetrodotoxin present in the final cooked dish, which add a unique tingle to the tip of the tongue when eaten. Of course where there is a lucrative gap for such a common hazard, there is extensive research being led by industrial chemists to finally create an antidote. Hopefully though, there are some ethical chemists who are researching in an attempt to finally put the minds of sushi lovers at rest as opposed to the monetary gains. [11]
Uses
Due to its ability to block sodium channels within neurones tetrodotoxin is used in neurologiacl research in the sudy of healthy and diseased neurones [12]. Tetrodotoxin has also found use as an anesthetic, with only 10mM being needed to produce an effect lasting up to eight hours[13].
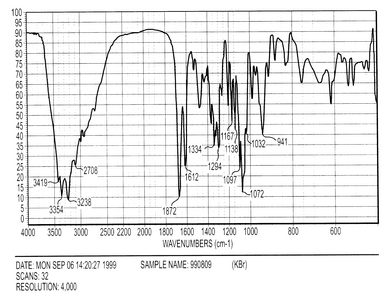
Spectra
Annotated IR Spectrum
References
- ↑ http://www.cfsan.fda.gov/~mow/tet.html
- ↑ Y. Kishi et al, J. Am. Chem. Soc.; 1972; 94(26) pp 9219 – 9221 DOI:10.1021/ja00781a039
- ↑ Hinman A, Du Bois J, . J. Am. Chem. Soc., 2003, 125 (38): 11510 -11511 DOI:10.1021/ja0368305
- ↑ Ohyabu N, Nishikawa T, Isobe M, J. Am. Chem. Soc.,2003, 125 (29): 8798-8805 DOI:10.1021/ja0342998
- ↑ Ohyabu N, Nishikawa T, Isobe M, J. Am. Chem. Soc.,2003, 125 (29): 8798-8805 DOI:10.1021/ja0342998
- ↑ Hinman A, Du Bois J, . J. Am. Chem. Soc., 2003, 125 (38): 11510 -11511 DOI:10.1021/ja0368305
- ↑ http://www.rsmas.miami.edu/groups/niehs/science/fugu.htm
- ↑ http://www.rsmas.miami.edu/groups/niehs/science/fugu.htm
- ↑ http://www.cfsan.fda.gov/~mow/chap39.html
- ↑ http://japanesefood.about.com/cs/seafoodfish/a/fugublowfish.htm
- ↑ Rivera V.R., Poli M.A., Bignami G.S., Toxican, 33:9 9/95 pg 1231-7
- ↑ http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/T5651
- ↑ http://sulcus.berkeley.edu/mcb/165_001/papers/manuscripts/_136.html
- ↑ A. Alcaraz, R. E. Whipple, H. R. Gregg, B. D. Andresen and P. M. Grant, Forensic Science International, 1999, 99, 35-45, DOI:10.1016/S0379-0738(98)00171-6
- ↑ Attained from 'Method of Extracting Tetrodotoxin' www.freepatentsonline.com/6552191.html