It07:Tetrahydrocannabinol
Tetrahydrocannabinol
| It07:Tetrahydrocannabinol | |
|---|---|
| |
| General | |
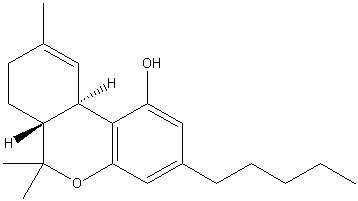
| Systematic name | (−)-(6aR,10aR)-6,6,9-trimethyl-
3-pentyl-6a,7,8,10a-tetrahydro- 6H-benzo[c]chromen-1-ol |
| Other names | THC, Dronabinol, Marinol, Deltanyne, Tetranabinex, Compassia, delta9-THC, delta1-THC, delta(sup 1)-Thc,delta(sup 9)-THC.[1] |
| Molecular formula | C21H30O2 |
| SMILES | CC1=C[C@]2([H])[C@@](C(C)(C) OC3=C2C(O)=CC(CCCCC)=C3)([H])CC1 |
| Molar mass | 314.45 gmol-1 |
| Appearance | |
| CAS number | 1972-08-3, 3556-79-4, 6087-73-6, 6465-30-1, 16849-50-6, 17766-02-8, 26906-54-7, 43009-38-7, 69855-10-3 |
| Properties | |
| Density & phase | {{{Density}}} g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | {{{Mp}}} K |
| Boiling point | 155-157°C DOI:10.1021/ja01062a046 [2] |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | MSDS |
| Main hazards | Extremely Flammable
Reacts violently with water Contact with water liberates extremely flammable gases Explosive when mixed with oxidizing substances Spontaneously flammable in air In use may form flammable/explosive vapor-air mixture May form explosive peroxides Harmful by inhalation Harmful in contact with skin Harmful if swallowed Toxic by inhalation Toxic in contact with skin Toxic if swallowed Danger of very serious irreversible effects [3] |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | HP8225000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Introduction
Tetrahydrocannabinol (commonly known as cannabis) is obtained by harvesting the resin of Cannabis sativa (marijuana, hashish). It is one of the oldest hallucigens in the world.It is a psychoactive compound, which triggers characteristic mood and perceptual changes in the user. There are many isomers, but the most active form is delta-9-tetrahydrocannabinol (THC), creating the most intense effects when used. [4] THC belongs to a sub-class of compounds called cannabinoids, which in turn belong to the class of molecules called terpenoids. Related to THC are menthol,turpentine and camphor[5].
3D Structure
Tetrahydrocannabinol |
Image was taken from http://en.wikipedia.org/wiki/Tetrahydrocannabinol
3D crystal structure (unit cell)
with (CH3)2N-CHO in lattice
Pentahelicene |
Synthesis
There is a long synthesis root for Tetrahydrocannabinol which can be found at: Synthesis[6] Pages 30-36 of the Original document. It is a long synthesis which has emphasis on the stereochemistry of the final product. This is because the drug has to interact with the body to have a desired effect and since the receptors are made out of chiral amino acids it must have the correct stereochemistry to interact.
Effects on the brain
THC effects the brain by binding to receptors in the brain which promote the release of Acetyl Choline[7] and Dopamine. There are various cannabinoid G protein-coupled receptors in the form of CB1 and CB2 as well as unusual receptors GPR55 and GPR119 which are needed to explain some of the other unexplainable pharmalogical effects of cannabinoids such as THC [8]. THC works to effect the brain in various ways as described next.
THC can cause change in levels of cAMP which in turn effect hyperpolarization-activated cyclic nucleotide-gated channels which then effects the ability of communication of brain cells.
[9]THC acts by binding to cannabinoid receptors and then causing the release or uptake of various neurotransmitters. Cannabinoid receptors are responsible for the control of thought, memory, concentration and movement. In the hippocampus there are a lot of THC receptors and the way information is taken in by this part of the brain is effected. The hippocampus is responsible for memory and so THC can lead to short term memory problems. Also the binding to the receptors causes damage to the nerve cells and so decreases their activities.
THC can also effects the emotions of the consumer by effecting the brains limbic system which controls behaviour and emotions. It is responsible for the characteristic laughing and/or paranoia associated with the intake of cannabis. Taking 14mg or more of THC at one time can have dramatic effects on the emotions and bodily functions of the person involved.
THC also gives the "feel good factor" which is made by the interaction of drug with the part of the brain which acts on reward. To do this the THC encourages the release of dopamine by the brain cells. Coordination is effected by the tetrahydrocannabinol by its binding with the receptors in the brain (cerebellum and basal ganglia). This has not been proven to cause accidents because any found THC levels in people that cause accidents are also usually accompanied with alcohol as well.
Brain areas in which cannabinoid receptors are abundant and hence effect to some extent:[10]
- Cerebellum (movement)
- Hippocampus (Memory)
- Cerebral cortex (Higher thought)
- Nucleus accumbens (Rewards)
- Basal ganglia (control of movement)
Less abundant(but still present) in:
- Hypothalamus (Regulation)
- Amygdala (Emotions)
- Spinal cord (Peripheral sensing)
Medicinal Uses
The delta-trans form of THC (also known as dronabinol) has anti-emetic effects (inhibits vomiting), thus making it a useful substance for cancer patients on chemotherapy. Also as well as reducing the vomiting response, it increases the appetite, thus making it a treatment for anorexia and other eating disorders [11]. Recent research has shown that this form of THC is a useful anti-glaucoma agent[12].
References
- ↑ http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=16078
- ↑ Y. Gaoni and R. Mechoulam, J. Am. Chem. Soc., 1964, 86, 1646-1647.
- ↑ http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/T4764
- ↑ http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=16078
- ↑ http://www.ch.ic.ac.uk/vchemlib/mim/bristol/thc/thc_text.htm
- ↑ Patent: Cabaj John E et al. Methods and Intermediates for the synthesis of Delta-9 Tetrahydrocannabinol, Pub no. WO2005100333, 30-36. (find document link in article)
- ↑ A. Pisanu, E. Acquas, S. Fenu and G. Di Chiara, Neuropharmacology, 2006, 50, 661-670. DOI:10.1016/j.neuropharm.2005.11.023
- ↑ A. J. Brown, Br J Pharmacol, 2007, 152, 567-575.DOI:10.1038/sj.bjp.0707481
- ↑ Serendip: http://serendip.brynmawr.edu/bb/neuro/neuro04/web1/aejelonu.html
- ↑ National Institute of Drug Abuse: http://www.nida.nih.gov/ResearchReports/Marijuana/Marijuana3.html
- ↑ http://www.ch.ic.ac.uk/vchemlib/mim/bristol/thc/thc_text.htm
- ↑ http://biotech.icmb.utexas.edu/botany/thc.html
