It07:Taurine
| It07:Taurine | |
|---|---|
| |
| General | |
| Systematic name | Taurine |
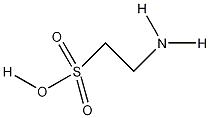
| Molecular formula | C2H7NO3S |
| SMILES | NCCS(=O)(O)=O |
| Molar mass | 125.14 g/mol |
| Appearance | White crystalline solid |
| CAS number | 107-35-7 |
| Properties | |
| Density | 1.734 g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | 578.26 K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | {{{Hazards}}} |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Taurine
The name taurine was derived from the latin name for ox, Bos Taurus. It was from an ox where taurine was first isolated in 1827. Taurine is a naturally occurring organic acid, which has various roles in the human body, but primarily it plays a major role in the formation of bile salts in the hepatocyte cells of the liver. Taurine is often referred to as an amino acid, but due to the lack of a carboxyl group this is not strictly true. Instead it may be called an amino sulfonic acid due to the presence of a sulfonate group. Also unlike most amino acids, the taurine molecule does not rotate polarised light, due to the lack of L- or D- configurations. In the body it occurs as a free molecule and is not incorporated into muscle proteins. The water soluble (hydrophilic) nature of taurine prevents it from crossing the body’s mostly fatty cell membranes however, it is present all cell membranes.
Physiological Roles
Taurine is conjugated via its terminal amino group with the bile acids chenodeoxycholic acid and cholic acid to form the bile salts sodium taurochenodeoxy cholate and sodium taurocholate. Bile salts break down fat globules in the duodenum through the process of emulsification.
Taurine is also associated with other physiological phenomena including inhibitory neurotransmission, long term potentiation in the hippocampus, adipose tissue regulation and calcium homeostasis.
Premature babies often lack the enzymes needed to convert cystathionine to cysteine and as a result may become deficient in taurine. When this is the case taurine is a dietary essential nutrient and is added to infant formulae.
Detoxification
Hypochlorous acid is a potent oxidizing substance which can cause severe damage to DNA. Taurine however can help to prevent this from happening due to the fact that the taurine structure contains a sulphonic acid moiety rather than a carboxylic acid one, it therefore does not form an aldehyde when it reacts with hypochlorous acid, instead it forms a relatively stable chloroamine compound. Therefore taurine acts as an antioxidant, regulating the concentrations of the chloride ion and the hypochlorous acid concentration, and also in the mean time it protects the body from the potentially toxic effects of aldehyde release.
Hormonal effects
In woman, taurine acts on the pituitary gland and increases the levels of the hormone prolactin into the blood. Prolactin acts upon the mammary glands causing them to lactate. Prolactin also promotes better fetal development.
Taurine production and excretion
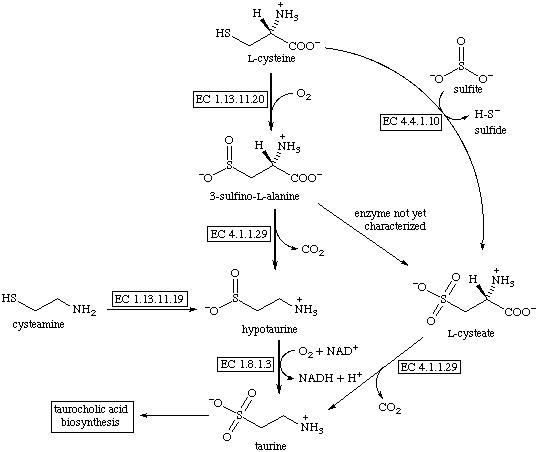
Taurine is formed primarily in the body from the amino acid cysteine. Active vitamin B6 is needed for this to occur. Excretion of taurine is reduced when there is a lack of vitamin B6 in the body, this suggests that adequate vitamin B6 intake is necessary for taurine production.
Taurine is excreted in two ways: If taurine levels in the body are adequate it is eliminated in the urine, if however the levels are too low the kidneys reabsorb it to prevent it from being lost. Taurine is also excreted in bile bound to bile salts.
Taurine and cats
Cats unlike humans cannot synthesise Taurine sufficiently in their body. The absence of Taurine in a cat’s diet causes the retina to slowly degenerate causing eye problems and eventually resulting in blindness. This is known as central retinal degeneration (CRD). CRD is most common in kittens. It can be reversed if the condition is caught early enough and if Taurine supplements are added to the cat’s diet.
Taurine and heart disease
Taurine in some countries is used to treat ischemic heart disease. Patients treated with Taurine (3-5g per day) were reported to have made an improvement in their condition. Research is also being carried out into the treatment of both hypertension and high cholesterol with Taurine.
Sources of taurine
Dietary taurine can be found in foods such as shellfish and organ meats i.e. liver. Human milk also contains taurine, but cows’ milk does not. Taurine is also one of the active ingredients found in energy drinks such as red bull, and in caffeine pills such as pro plus.