It07:Strychnine
| It07:Strychnine | ||
|---|---|---|
| ||
| General | ||
| Name | Strychnidin-10-one | |
| Molecular formula | C21H22N2O2 | |
| SMILES | ||
| Molar mass | 334.41 g/mol | |
| Appearance | Colourless liquid | |
| CAS number | 57-24-9 | |
| Properties | ||
| Density & phase | {{{Density}}} g/cm³ | |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) | |
| Melting point | ||
| Boiling point | 286-288oC (559-561K) | |
| Acidity (pKa) | {{{pKa}}} | |
| Basicity (pKb) | {{{pKb}}} | |
| Chiral rotation [α]D | {{{Rotation}}}° | |
| Viscosity | {{{Viscosity}}} cP at 25°C | |
| Structure | ||
| Molecular shape | {{{Mol_Shape}}} | |
| Coordination geometry |
{{{Coordination}}} | |
| Crystal structure | {{{Crystal_Structure}}} | |
| Dipole moment | {{{DM}}} D | |
| Hazards | ||
| MSDS | External MSDS | |
| Main hazards | {{{Hazards}}} | |
| NFPA 704 | {{{NFPA}}} | |
| Flash point | {{{Fp}}}°C | |
| R/S statement | R: {{{R-S}}} S: ? | |
| RTECS number | {{{RTECS}}} | |
| Supplementary data page | ||
| Structure and properties |
n, εr, etc. | |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas | |
| Spectral data | UV, IR, NMR, MS | |
| Related compounds | ||
| Other anions | {{{Other_anion}}} | |
| Other cations | {{{Ohter_cation}}} | |
| Related compounds | {{{Relative_Compounds}}} | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Vas_strychnine.pdb |
Introduction
Strychnine is an alkaloid molecule found in the seeds of the plant Strychnos Nux Vomica, native to India, Sri Lanka and Australia. It is a toxic substance that can cause fatality in humans and is one of the most bitter substances yet to be discovered. It can be inhaled, ingested or absorbed through the eyes or mouth. The effects can be felt as soon as 10 minutes after exposure, when muscles spasms begin to occur in the head and neck. Convulsions then begin throughout the body, paralysing the neural system and causing asphyxiation after approximately 2 hours.
Mechanism of Action
In synapses in the central nervous system there are two functionaly different types of receptor. Binding at stimulatory receptors increases the signal transmittance likelihood, while binding at inhibitory receptors reduces chances. Glycine receptors are one of the main types of inhibitory receptor, particularly in the lower parts of the central nervous system, and it is these that are blocked by strychnine.
The result is huge over-transmission of signals resulting in reflex arcs, which would normally be suppressed by the postsynaptic action of glycine. This means that the tiniest sensory stimulus produces powerful muscular contraction.
This leads to powerful and extremely painful convulsions. Death occurs as a result of respiratory arrest, due to spasm and paralysis of the respiratory muscles.
Symptoms usually begin within about 20 minutes of ingestion, but it can be more gradual. The lethal dose is about 5mg/kg body-weight, in other words about 350mg for an adult.[1]
History
Other than its obvious use by assassins as a deadly poison strychnine has been used in the past to treat appetite loss. The bitter taste stimulates salivary and gastric enzyme secretions, combatting loss of appetite. This approach was used in the 19th century (with miniscule amounts), however these effects were generally overwhelmed by the poisonous symptoms, which resulted in severe illness for many patients, resulting in its diminished use subsequently, and its complete absence as a remedy today.
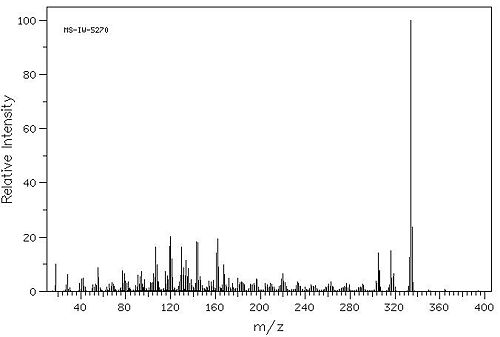
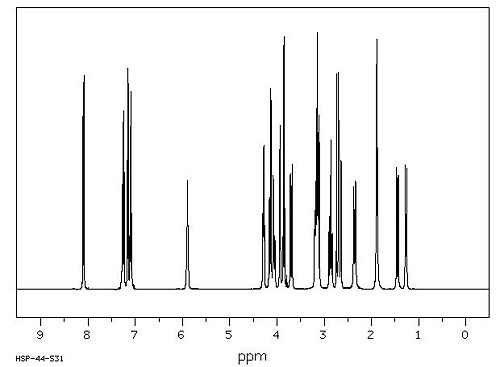
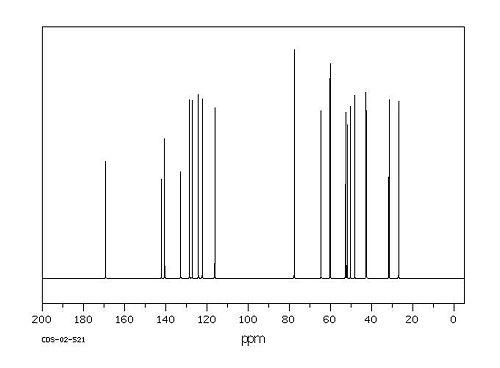
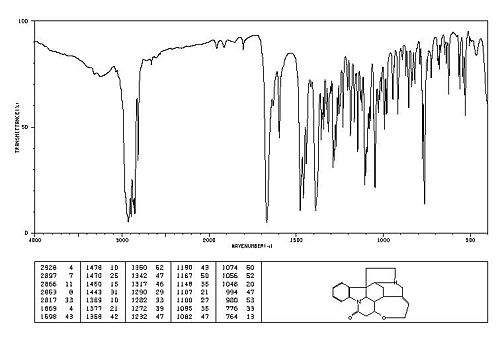
Spectroscopic Data[2]
Mass Spectrum
1H NMR Spectrum
13C NMR Spectrum
Infra-Red Spectrum
References
- ↑ http://www.portfolio.mvm.ed.ac.uk/studentwebs/session2/group12/strychni.htm
- ↑ AIST:PIO-D8 Spectral Database for Organic Compounds, SDBS