It07:Simvastatin
Simvastatin belongs to a group of drugs that inhibit the rate limiting enzyme of cholesterol synthesis in the body HMG-CoA reductase. It is especially useful in treating hypercholesterolemia a name given to a condition that is associated with high levels of cholesterol, in particular LDLs, in the blood.
3d Structure
| Template:Simvastatin | |
|---|---|
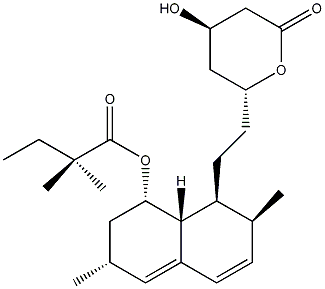 | |
| General | |
| Systematic name | 2,2-dimethyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-naphthalen-1-yl ester
|
| Other names | Colemin, Lipex, Pantok, Synvinolin |
| Molecular formula | C25H38O5
|
| SMILES | O[C@@H]1C[C@@H](CC[C@@H]2[C@]3([H])C(C=C[C@@H]2C)=C[C@H](C)C[C@@H]3OC([C@](C)(C)CC)=O)OC(C1)=O |
| Molar mass | 418.57
|
| Appearance | white solid |
| CAS number | 79902-63-9 |
History
[1] The first HMG-CoA reductase inhibitor was discovered in 1973 by Akira Endo in Japan and was named mevastatin. Simvastatin is an analogue of this compound, and was chemically synthesised from lovastatin, another analogue of mevastatin, and launched in 1988 by Merck Co. in the US.
Applications
[2]Simvastatin lowers cholesterol in the body by inhibiting its production in the liver. In addition to lowering overall cholesterol, it targets in particular coronary heart disease causing LDLs (low density lipoprotein). Therefore the main use for Simvastatin is for treatment of high blood cholesterol as a secondary treatment to exercise, weight loss and a low cholesterol diet.
Side Effects
[3] As with any drug, simvastatin also has side effects. Some common side effects include diarrhea, abdominal pain and indigestion and some of the less common side effects include memory loss, muscle cramps and joint pain. As is the case with many drugs, the only way to tell which, if any, of the side effects will be developed in an individual is to actually take the drug.
Synthesis
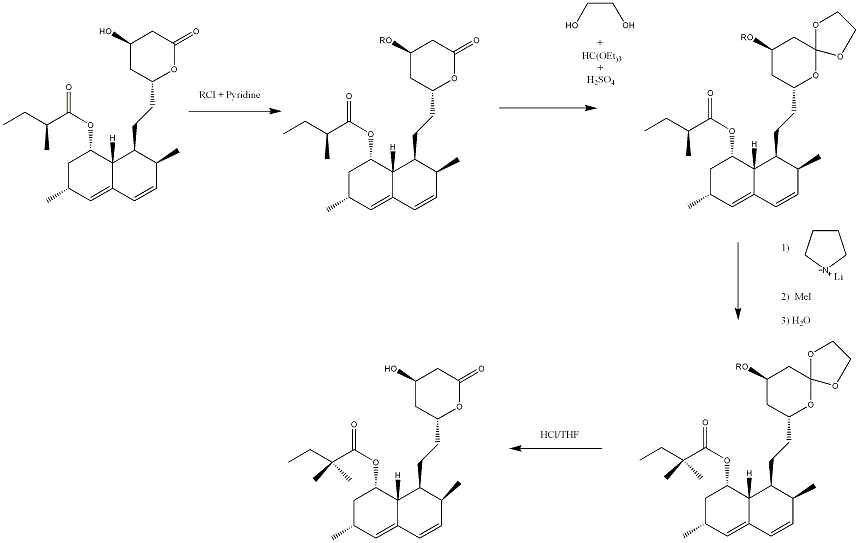
[4]Simvastatin can be safely and reliably synthesised from another compound that is also a statin, known as lovastatin. Although the starting reactant is the same as the final product apart from an extra methyl group, successful methylation of only that carbon and not the lactone C=O group(on the ring) depends on the introduction of a C=O protecting group. This, in addition to the introduction of an OH protecting group as well as the removal of these groups, accounts for the steps that are extra to the methylation of the side chain.
In the first step lovastatin is treated with RCl and pyridine in a CHCl2 solvent where R may be PhCOCl or t-BuCO. This gives compound 2 which, in THF solvent, then has added to it ethane-1,2-diol, (EtO)3CH and an amount of H2SO4 that is catalytic. This gives the third compound known as an orthoformate. In the third step, this compound is treated with BuLi, MeI, pyrrolidine and water leading to the methylation of its (S)-2-methylbutanoyloxy side chain. The final compound, simvastatin, is obtained by removal of the C=O protecting group, by means of treating with dilute HCl in THF solvent, restoring the carbonyl group that was originally in that position in the starting product.
Spectroscopy
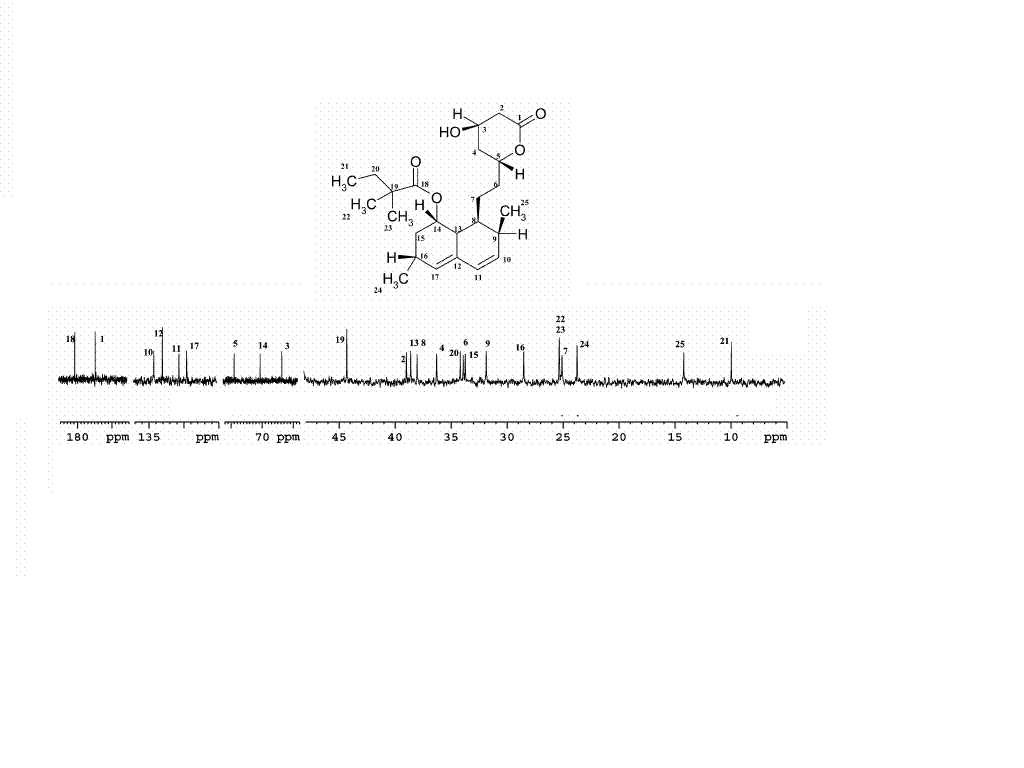
[5]A 13C NMR spectrum of Simvastatin
References
- ↑ p132 Innovation in the Pharmaceutical Industry: The Process of Drug Discovery and Development... By Takuji Hara [1]
- ↑ http://www.medicinenet.com/simvastatin/article.htm
- ↑ Simvastatin Side Effects: An Introduction[2]
- ↑ DOI:10.1002/hlca.200390066
- ↑ DOI:Phys. Chem. A, 108 (18), 3955 -3964, 2004. 10.1021/jp0498163 S1089-5639(04)09816-0 J. Phys. Chem. A, 108 (18), 3955 -3964, 2004. 10.1021/jp0498163 S1089-5639(04)09816-0
