It07:Sibutramine
| Sibutramine | |
|---|---|
[[Image: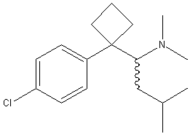 |250px|Sibutramine]] |250px|Sibutramine]]
| |
| IUPAC Systematic name | |
| 1-[1-(4-chlorophenyl)cyclobutyl]-N,N,3-trimethylbutan-1-amine | |
| Other name | |
| Reductil&btnG=Google+Search&meta= Meridia, Reductil | |
| Indentifiers | |
| ATC Code | A08AA10 |
| CAS number | {{{CASNo}}} |
| PubChem (CID) | 5210 |
| SMILES | in nowiki script code '<' nowiki'>' insert SMILE here'</'nowiki'>' surround in nowiki script code '<' nowiki'>' insert SMILE here'</'nowiki'>' |
| Chemical Data | |
| Molecular formula | C17H26ClN |
| Molar mass | 279.84804g/mol g/mol |
| Pharmacokinetic Data | |
| Bioavailability | Resorption 77%, considerable first-pass metabolism |
| Protein Binding | {{{Protein_binding}}} |
| Metabolism | Hepatic |
| Half life | sibutramine approx. 1 hour
Metabolite 1: 14 hours Metabolite 2: 16 hours |
| Excretion | Biliary (sibutramine and active metabolites), renal (inactive metabolites) |
| Therapeutic considerations | |
| Pregnancy cat. | X, no human data existing, teratogenic potential in animal studies |
| Legal status | |
| Routes | Oral |
3D Model of Sibutramine
Sibutramine |
General Information
Sibutramine has been used as an appetite suppressant since it was approved by the FDA in the US in 1997. It is produced and marketed by Knoll Pharmaceuticals and is available in over 40 countries.
It was originally evaluated as an antidepressent due to its mechanism action being similar to the tricyclic group of antidepressents, such as Amitriptyline without having the same negative side effects as Amitriptyline
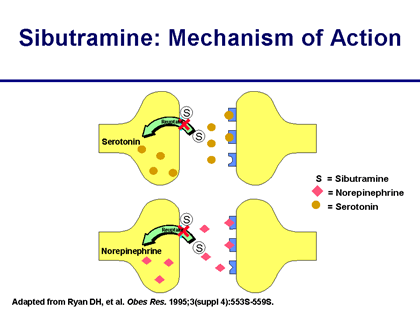
Sibutramine is found in a class of medications called appetite suppressants. Sibutramine is commonly used in conjunction with a reduced calorie diet and exercise to help overweight people diminish their appetite. It primary mechanism involves acting on neurotransmitters that control appetite in the brain to decrease appetite.
History
Sibutramine was originally investigated as an anti-depressent due to its action as a serotonin uptake inhibitor. Seratonin (structure shown below) is a neurotransmitter, which is responsible for the feeling of ‘happiness’. The body controls the levels of seratonin very carefully and when levels get too high, it is reabsorbed. By inhibiting this reabsorption, the levels are able to get a little higher allowing the person a constant feeling of wellbeing. It showed real potential as a new drug as it did not seem to have any of the negative side effects usually associated with anti-depressents.
It was through the trials of sibutramine as an anti-depressent that it was discovered that patients also showed significant weight loss. It, therefore, started to become investigated as a possible anorexiant. Following this discovery, sibrutamine’s properties as an anti-depressent stopped being evaluated, perhaps due to a lack of positive or consistent results, and its properties as an anorexient became the subject of new research. Sibutramine is now marketed in its HCl monohydrate form as MeridiaTM and ReductilTM
How does it work?
Sibutramine specifically blocks the reuptake of the neurotransmitters dopamine, norepinephrine, and serotonin. These are brain chemicals which influence feelings of hunger and fullness and taking sibutramine will induce the sensation of feeling full sooner when you eat and thus the patient will eat less.
Pharmacokinetics
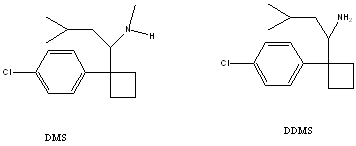
Sibutramine is absorbed (77% of a single dose) in the gastro-intestinal tract and, first, is metabolised in the liver by the cytochrome P450(3A4) to DMS and DDMS (structures shown below). The half life of this is just over 1 hour, and peak plasma concentrations of the metabolites are then reached after 3 to 4 hours. DMS and DDMS are then further metabolised in the liver by hydroxylation to pharmacologically inactive forms, with elimination half lives of 16 and 14 hours respectively. Sibutramine is excreted over approximately 15 days (85% of a dose), 77% of which is excreted in the urine.
Side effects
The most common side effects that occur whilst a patient is taking sibutramine are as follows; dry mouth, headache, constipation and insomnia. The drug has also shown to cause a significant increase in heart rate and diastolic blood pressure and, in some cases, persistent increases. Please see 'Further Studies'.
Reaction Mechanism for the Synthesis of Sibutramine

Further Studies
In 2002 a petition was submitted to the FDA declaring the use of sibutramine to be banned. http://www.citizen.org/publications/print_release.cfm?ID=7160 It was reported that the use of sibutramine had been associated with 29 deaths including 19 from cardiovascular adverse effects in people using this drug. Previously its use was even suspended in Italy because of two cardiovascular deaths. The petition refers to clinical trials where it was shown that taking the drug resulted in a significant increase in blood pressure and heart rate.
Other research, http://www.rxlist.com/cgi/generic/sibutramine_ad.htm, indicated that Convulsions were reported as an adverse event in three of 2068 (0.1%) sibutramine treated patients. There were also abnormal liver function tests. Cases of depression, suicidal ideation and suicide have been reported rarely in patients on sibutramine treatment. However, a relationship has not been established between the occurrence of depression and/or suicidal ideation and the use of sibutramine.
References
- ↑ Sibutramine History - http://www.chemsoc.org/ExemplarChem/entries/2003/imperial_Rowlands/web/history.html (Provided by Imperial College)
- ↑ http://www.vaccine-edu.com/public/2006-201/slides/Slide34.gif
- ↑ Sibutramine Clinical - http://www.chemsoc.org/ExemplarChem/entries/2003/imperial_Rowlands/web/pharmacology.html (Provided by Imperial College)
- ↑ Jeffery, J. E. J. E. (1996). "Synthesis of sibutramine, a novel cyclobutylalkylamine useful in the treatment of obesity, and its major human metabolites." Journal of the Chemical Society. Perkin transactions 1(21): 2583-2589.