It07:Schwartz
| It07:Schwartz | |
|---|---|
 | |
| General | |
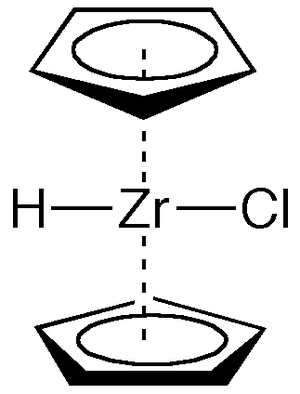
| Systematic name | Bis(η5-cyclopentadienyl)- zirconium(IV) chloride hydride |
| Other names | Cp2ZrClH, zirconocene chloride hydride |
| Molecular formula | C54H45ClP3Rh C10H11ClZr |
| Molar mass | 257.87 g/mol-1 |
| Identifier | |
| CAS number | 37342-97-5 |
| Properties | |
| Appearance | White Solid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Schwartz reagent
Schwartz's reagent is the commonly used name for the chemical compound with the formula (C5H5)2ZrHCl. This metallocene is used in organic synthesis for many transformations of alkenes and alkynes. Schwartz's Reagent is very sensitive to air, moisture, and moderately sensitive to light sensitive. Therefore, it should be stored in the dark, dry place.[1]
3D Model of Schwartz reagent
Sibutramine |
Uses
Schwartz's reagent is used as a reagent for the functionalization of olefins and alkynes and it is also used in conversion of amides to aldehydes. [2]
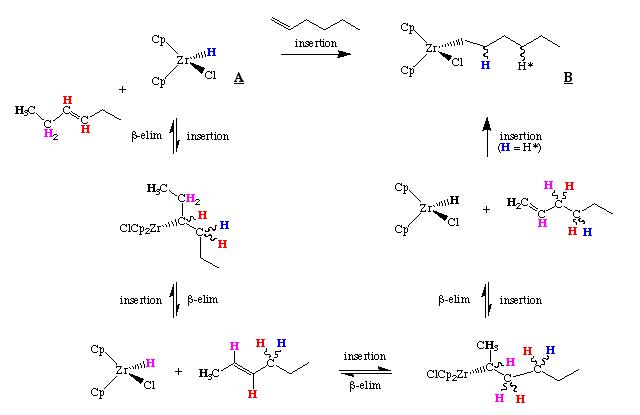
Hydrozirconation reaction
Schwartz's reagent reacts with alkenes and alkynes via called hydrozirconation. This reaction results in the addition of the Zr-H bond across the C=C or C≡C bond. In this reaction the insertion of either a terminal or internal olefin gives the same product. The order of the olefin reactivity is terminal alkyne > terminal alkene ~ internal alkyne > disubstituted alkene. This isomerization occurs through a series of beta-hydride elimination and olefin insertion reactions as shown below. [3]
IR spectrum