It07:Schrock
The 2005 Chemistry Nobel Prize was awarded jointly to Robert Schrock, Yves Chauvin and Robert Grubbs, as they pioneered cleavage of the carbon-carbon double bond. The metathesis catalysis is used for olefins (olefin is another name for an alkene), or alkylidenes, specifically, and has various uses and applications, including in industry. There are various different Shrock metathesis catalysts, all with the standard formula [M]=CHR.
The Schrock metathesis catalysts are a series of complexes of molybdenum and tungsten-alkylidenes. The original series of Schrock metathesis catalysts were unstable and oxygen and water sensitive. A group led by Robert Grubbs found that similar molecules with a ruthenium centre were not sensitive to water and had greater functional group tolerance.
Over the last ten years, as they have become more and more developed, homogeneous catalysts such as Schrock’s catalyst (A) and Grubbs’ catalyst (B)[1] have become increasingly important in the synthesis of many organic molecules including macrocyclic rings and polymers of olefins:
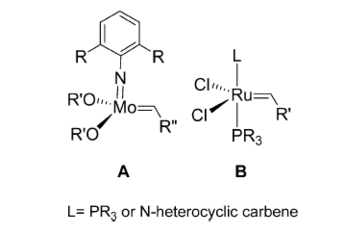
This is down to the enabling of the process of olefin metathesis:
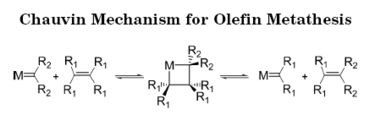
Schrock metathesis catalyst
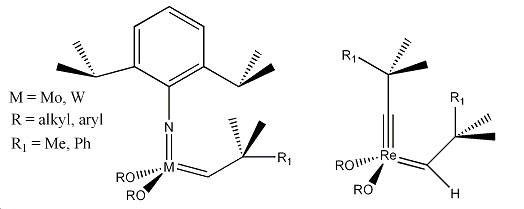
General formula for a Schrock metathesis catalyst:
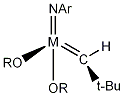
Commercially available example of a Schrock metathesis catalyst
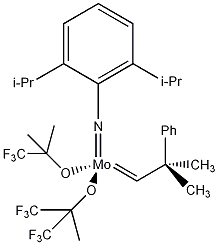
3D Representation of a Generic Schrock Metathesis Catalsyst
(2,6-Dimethylphenylimido)-bis(t-butoxy)-(2-methyl-2-phenylprop-1-ylidene)-Molybdenum(VI)
| ||||
|---|---|---|---|---|
References
- ↑ T.P.M. Goumans, A.W. Ehlers, and K. Lammertsma, Am. Chem. Soc., 2005, 24, 3200-3206
