It07:Sarin
Sarin
2-(fluoro-methyl-phosphoryl)oxypropane, also known as Sarin, is an extremely toxic substance classifed by the United Nations as a weapon of mass destruction. Its principality is a nerve agent, and it is said that just a drop the size of a pinhead is more than adequate to kill a person. [1] It derives its name from the synthetic chemists who were responsible for creating it - Schrader, Ambrose, Rüdige, and van der Linde.
| It07:Sarin | |
|---|---|

| |
| General | |
| Systematic name | 2-(fluoro-methyl-phosphoryl)oxypropane |
| Other names | Sarin, O-Isopropyl methylphosphonofluoridate |
| Molecular formula | C4H10FO2P |
| SMILES | O=[P@](F)(OC(C)C)C |
| CAS number | - |
| Properties | |
| Molar mass | 140.04gmol-1 |
| Melting point | -56°C |
| Boiling point | 158°C |
| Appearance | Clear colourless liquid. Odourless in pure form. |
Sarin |
History
The chemical, Sarin, was one of the group of nerve agents invented by a group of German scientists in the 1930s as part of Hitler's preparation for World War II. However, the Germans decided not to incorporate the usage of Sarin against Allied troops for fear that the Allies would develop their own chemical weapons that could prove devastating in an all-out chemical warfare.
During the 1960s, the United States and USSR secretly built huge stockpiles of Sarin, in times when the Cold War escalated to unprecedented levels of fear.
Although the Germans never released Sarin during battle, one of the well known cases of usage of sarin to lethal effect was by Iraq, on Iran and the Kurds during the 1980s. Another well known publicised case was the Sarin attack on a Tokyo subway during the 1950s when members of an apocalyptic cult group, Aum Shinrikyo, released the deadly gas on several lines of the Tokyo subway, ultimately killing 12 people.
Synthesis of Sarin
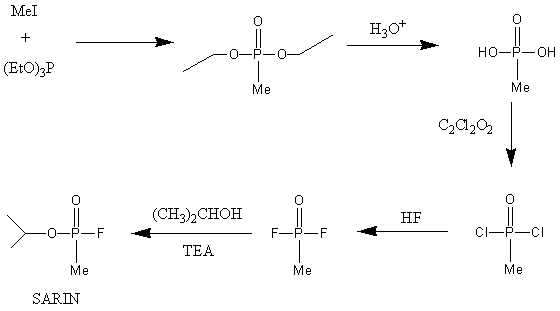
One method of synthesising Sarin is by following a 5-step sequence, as shown by the reaction scheme below:
Triethyl phosphite and methyl iodide are reacted in the Michaelis-Arbusov reaction to produce diethyl methyl phosphonate which is converted to methylphosphonic acid by hydrolysis. After chlorination and subsequent fluorination, the final product is formed by reaction with the appropriate alcohol.
The sequence generates by-products that can be easily removed, as well as creating favourable yields ~80-90% [2]
Biological Effects
Sarin is an irreversible chlorinesterase inhibitor that operates by attacking the nervous system of a living organism. As an organophosphate compound, its functionality is very similar to that of insecticides - primarily, it inhibits the enzyme acetylcholinesterase by binding covalently to the serine residue, thereby reducing acetylcholine's ability to break down. In mechanistic terms, the fluorine on the phosphonyl group reacts with the hydroxyl group of the serine side chain, forming an phosphoester which subsequently releases HF.
The inhibition of the enzyme coupled with the increasing buildup of acetylcholine in the synapse means that the nerve impulses are being continually transmitted and received by organs/muscles. This causes a wide range of short-term and long-term symptons upon exposure with Sarin.
[3]Short term exposure symptons include:
- Runny nose
- Watery eyes
- Small, pinpoint pupils
- Blurred vision
- Drooling and excessive sweating
- Cough
- Difficulty breathing
- Diarrhea
- Increased urination
- Confusion
- Drowsiness
- Weakness
- Headache
- Nausea/vomiting
- Slow or fast heart rate
- Low or high blood pressure
Long term exposure symptons include:
- Loss of consciousness
- Convulsions
- Coma
- Death