It07:Saccharin
Saccharin
| It07:Saccharin | |
|---|---|
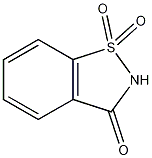
| |
| General | |
| Systematic name | 1,1-Dioxo-1,2-benzothiazol-3-one |
| Other names | Benzoic sulfinide; E954 |
| Molecular formula | C7H5NO3S |
| SMILES | O=C1NS(=O)(C2=C1C=CC=C2)=O |
| Molar mass | 183.18 gmol-1 |
| CAS number | 81-07-2 |
| Properties | |
| Melting point | 228.8-229.7 oC |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Introduction
Saccharin is the oldest known sugar substitute. Saccharin has the additive number E954. Saccharin is the foundation for many low-calorie and sugar-free products around the world. It is used in tabletop sweeteners, baked goods, jams, chewing gum, canned fruit, candy, dessert toppings and salad dressings. It also is useful in cosmetic products, vitamins and pharmaceuticals.
History
It was discovered at Johns Hopkins University in 1879, in the days before disposable gloves. Ira Remsen asked research fellow Constantin Fahlberg to oxidise a sulphonamide. Fahlberg did so and found that evening that the food he was eating tasted remarkably sweet. Sacharrin’s use was fairly limited until the two World Wars, when sugar shortages led to a huge demand for a sugar substitute. It also gained popularity during the 1960’s and 70’s with dieters, and through it’s use in diet soft drinks such as Tab, manufactured by Coca Cola.
Safety
Saccharin has been the subject of extensive scientific research since it’s introduction. Various studies have been conducted investigating the link between Saccharin and cancer, some showing a correlation between saccharin consumption and increased frequency of cancer (especially bladder cancer) and others finding no such correlation. No study has ever shown a clear causal relationship between saccharin consumption and health risks in humans at normal doses, though some studies have shown a correlation between consumption and cancer incidence. Theodore Roosevelt is famously quoted defending Saccharin "Anybody who says saccharin is injurious to health is an idiot."
Spectra
Infrared
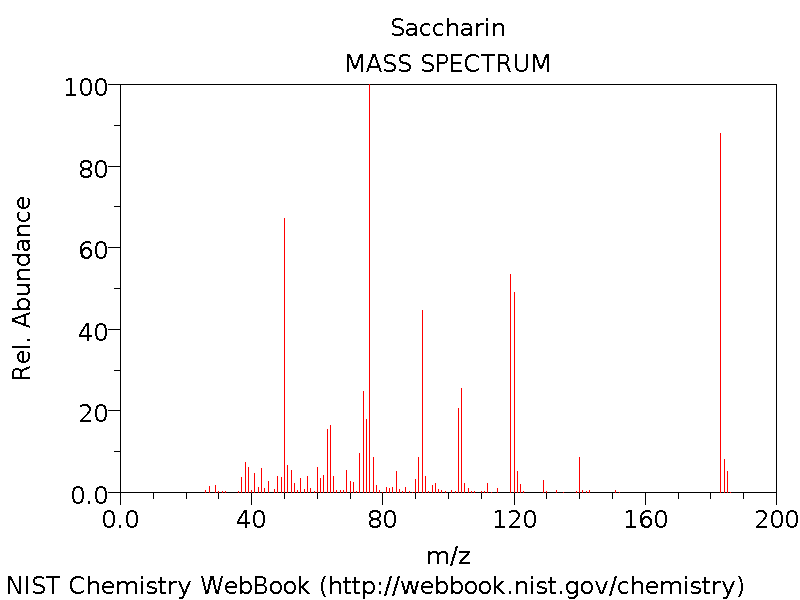
Mass Spectrum
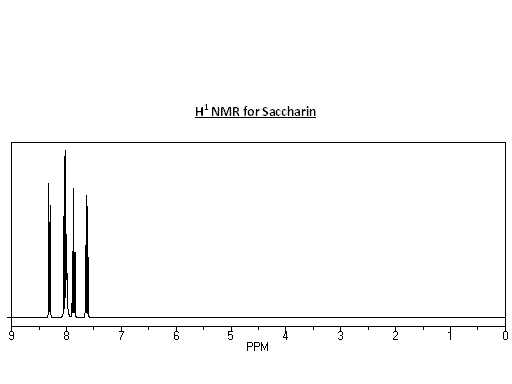
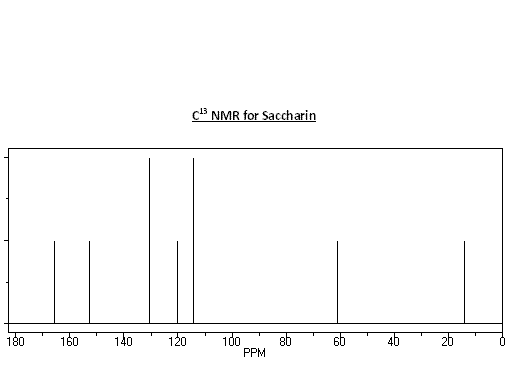
NMR
References
- "Organic Chemistry" Clayden et al
- http://webbook.nist.gov/chemistry/
- http://www.saccharin.org