It07:Rivaroxaban
| It07:Rivaroxaban | |
|---|---|
| |
| General | |
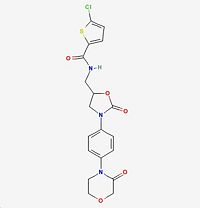
| Systematic name | 5-chloro-N-[[2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide |
| Other names | Xarelto, BAY-59-7939 |
| Molecular formula | C19H18ClN3O5S
|
| Molar mass | 435.88132 g/mol |
| Appearance | |
| CAS number | 366789-02-8 |
| Chemfinder ACX number | |
| SMILES | ClC1=CC=C(C(NCC(CN2C3=CC=C(N(CCOC4)C4=O)C=C3)OC2=O)=O)S1 |
| Properties | |
| Melting point | 503 K |
| Structure | |
| Molecular shape | Heterocyclic |
| Hazards | |
| Main Hazards | |
| NFPA 704 | |
| Flash point | N/A |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Rivaroxaban
Rivaroxaban, or Xarelto, is a drug used to prevent or treat blood clots (it is an anti- coagulant) by inhibition of Factor Xa- an enzyme found in the coagulation cascade. Although still under development by Bayer, test results suggest that Rivaroxaban is more effective than current medication in use.
Mode of action
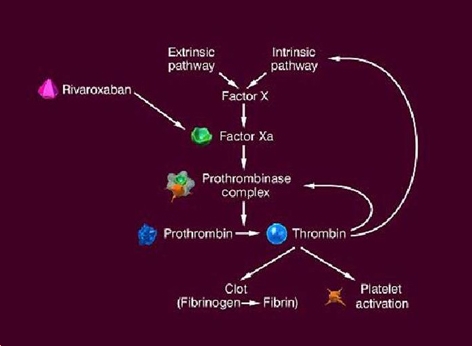
Rivaroxaban targets the active site of Factor Xa. Factor Xa is central in the coagulation cascade, connecting the intrinsic and extrinsic pathways. Factor Xa cleaves prothrombin to thrombin, which in turn catalyses the formation of fibrinogen to fibrin and thereby forming blood clots. Factor Xa is the only enzyme capable of cleaving prothrombin, thus blocking its action will undoubtedly stop blood clotting.
Clinical Trials
Extensive testing of Rivaroxaban against existing drugs seem to indicate that Rivaroxaban has a few advantages over current medication. Tests conducted using Rivaroxaban have been primarily related to its effectiveness against venous thromboembolism (VTE). Over 40,000 patients have been tested. Test results have shown that it has similar efficacy to the existing drug enoxaparin in prevention of VTE in patients undergoing knee replacement surgery. Other results have shown that the drug has predictable coagulation patterns, meaning that constant monitoring of dosage is not necessary- an advantage over using alternative drugs such as warfarin. Furthermore, it is an oral drug, dispensing the need for drug administration via injection (enoxaparin has to be administered in this way). Also, studies show that Rivaroxaban doesn’t interact with other drugs given in conjunction with anticoagulants.
Pentahelicene |
Pentahelicene |
