It07:Piperine
| It07:Piperine | |
|---|---|

| |
| General | |
| Systematic name | 1-(5-benzo[1,3]dioxol-5-yl-penta-2,4-dienoyl)-piperidine |
| Other names | 1-piperinoyl-piperidin; piperine |
| Molecular formula | C17H19NO3 |
| SMILES | O=C(/C=C/C=C/C2=CC3=C(OCO3)C=C2)N1CCCCC1 |
| Molar mass | 285.34 |
| Appearance | {{{Appearance}}} |
| CAS number | 94-62-2 |
| Properties | |
| Density & phase | 1.193 g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | 129-130°C K |
| Boiling point | {{{Bp}}} K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Hazards | |
| MSDS | Piperine MSDS |
| Main hazards | Irritant of eyes and skin |
| RTECS number | TN2321500 |
Piperine 3D Model
Right click on the above 3D molecule to explore more about its structure.
Overview
Piperine is a heterocyclic alkaloid found naturally in plants belonging to the Piperaceae family, such as piper nigrum L, commonly known as black pepper, and piper longum L, commonly known as long pepper. Piperine - comprising 1 to 99% of these plants - is responsible for the pungency of this family and is isolated from the fruit of the black pepper and long pepper plants. The term 'black pepper' is used both for the plant piper nigrum and the spice, which occurs mainly in the fruit. Piperine is a solid substance essentially insoluble in water. It is a weak base that is tasteless at first, but leaves a burning aftertaste. Piperine belongs to the vanilloid family of compounds, a family that also includes capsaicin, the pungent substance in hot chili peppers.
Aside from its culinary uses, Piperine has long been used for its medicinal properties in India. It has antiasthmatic, antifertility, CNS depressant and anti-inflammatory properites. It also inhibits the enzymes hepatic monooxygenase and UDP–glucoronyl transeferase.

History of Piperine
Piperine has had a great influence on food storage all over the world for thousand of years. Before the invention of processed foods and fridges, the possibilities for storing food were limited to drying or salting. However, if these did not work then the food would begin to go bad. Thanks to the strong flavour of the Piperine, pepper could be added to most foods to mask the taste.
Piperine was identified in 1820 by the Danish physicist, chemist and professor at the University of Copenhagen, Hans Christian Orsted. In 1882 and 1894, Piperine was successfully synthesized in the lab. Today the popularity of pepper is still very high and it is heavily used all over the world.
Substance Identification
- Autoname: 5-benzo[1,3]dioxol-5-yl-1-piperidin-1-yl-penta-2,4-dien-1-one
- Type of Substance: Heterocyclic
- Type of Bonding: Piperine has predominantly covalent bonds. However, with its large amount of hydrogen atoms it has hydrogen bonds as well.
- Melting Point
| VALUE(MP) | Solvent (.SOL) | Entry Date | Notes | Refs. |
|---|---|---|---|---|
| C | ||||
| 129.2 - 130.5 | 1 | |||
| 129 | diethyl ether | 2-3 | ||
| 128 | 4 | |||
| 128 - 129.5 | 5-6 | |||
| 129 - 130 | 7 | |||
| 128 - 129 | 8 | |||
| 127 - 129 | 9 | |||
| 128 - 129 | 10 | |||
| 120 - 129 | 11 |
- Purity in Nature: 98% in piper nigrum. Can only be attained in 100% purity through processing in the laboratory.
- Percent Composition by mass: C= 71% O= 17% H=6% N=5%
Spectra
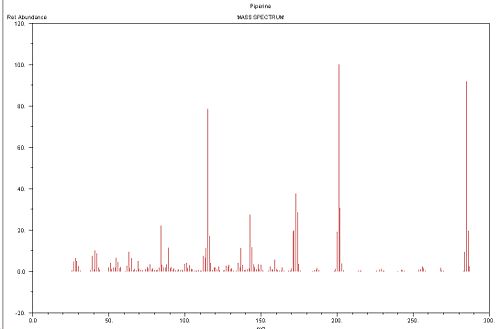
Mass Spectrum[1]
IR Spectrum[2]
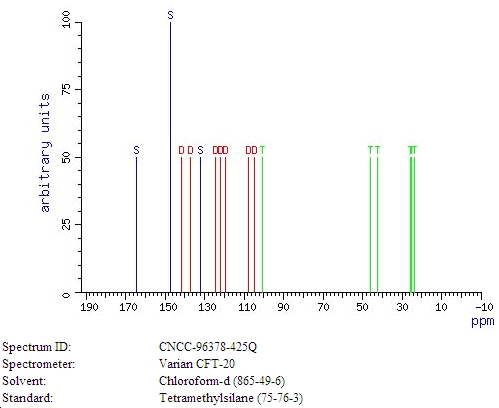
13C NMR[3]
Chemical properties
Piperine is a yellowish power in nature, turning to a stronger green tint after synthesis. Tasteless and unreactive unless in solution, it is soluble in alcohol, chloroform, ether, benzene and water. Its flavour comes from the reaction between the oil in the peppercorns and itself.
The transformation of Piperine into Chavicine is associated with the loss of the Piperine’s characteristic flavour. In a boiled alcoholic caustic potash, piperic acid can be produced from the Piperine.
Synthesis
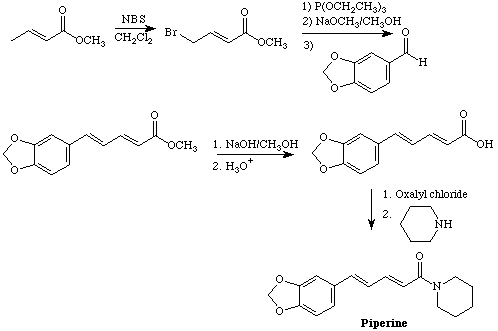
The synthesis starts with an allylic, radical halogenation to introduce a bromine atom. An Arbuzov reaction with triethyl phosphite displaces the bromine and produces a phosphonate that is then coupled to piperonal using a modified Wittig reaction. Hydrolysis of the ester, formation of the acid chloride using oxalyl chloride, and coupling to piperidine completes the synthesis.
Uses of Piperine
Piperine has been used for thousands of years to treat many small ailments. From recent medical studies, Piperine was found to greatly aid the absorption of certain vitamins (e.g. selenium, beta-carotene and vitamin B). In addition, Piperine also has the ability to increase the thermogenesis of a body, and create a demand for nutrients for metabolism as a consequence. This has a significant positive effect on the sick or aged with defective intestinal lining.
Country use: Mexico - anti-inflammatory, anti-malarial, cure for stomach ache Morocco - weight loss treatment, anti-leukemia treatment Indonesia - fever prevention/reducing, treatment for snake bites, anti-epilepsy treatment
However, Piperine can also be found in most insecticides.
Hazard identification
Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation (lung irritant).
Piperine may be combustible at high temperature.
Products of its combustion are potentially harmful as they contain CO, CO2, NO, NO2.
Precautions
Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Do not ingest or breathe dust. Avoid contact with skin and eyes.
Storage
Keep container dry. Keep in a cool, well-ventilated place. Keep container tightly closed.
References
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C94622&Units=SI&Mask=200#Mass-Spec
- ↑ AIST: Integrated Spectral Database System of Organic Compounds (Data were obtained from the National Institute of Advanced Industrial Science and Technology (Japan))
- ↑ WSS: Spectral data were obtained from Wiley Subscription Services, Inc. (US)
4. Sloop, J.C. J. Chem. Ed. 1995, 72, A25-A27 (Synthesis)
5. http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrugs/pip_0322.shtml
6. Substance Identification: Beilstein Reference 5-20-03-00469, 6-20
7. Melting Point 1-9 (Beilstein(2007/03): http://localhost:16001/x_BS070300AE_S90739.html
8. Little History of Piperine: http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2000/Piperine/piperin_history_page.htm
9.http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2000/Piperine/piperin_properties_page.htm
10.http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2000/Piperine/piperin_uses_page.htm
11.http://webbook.nist.gov/cgi/cbook.cgi?ID=C94622&Units=SI&Mask=200#Mass-Spec