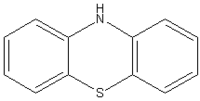
It07:Phenothiazine
Phenothiazine
Phenothiazine and derivatives have a tremendous range of uses from pharmaceutical applications to organic light emitting diodes. Phenothiazine is three-ringed system consisting of two benzene rings joined by a nitrogen sulphur ring.
Properties [1][2]
|
| |||||||||||||||||||||||||||||||||||||||||
Synthesis [3]
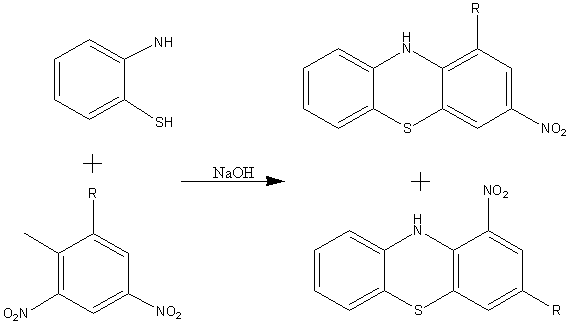
The Bernthasen synthesis (scheme 1) involves the heating of diphenyl amine and sulphur in high boiling point solvents. This forms a basic phenothiazine. Scheme 2 shows the preparation of a phenothiazine by basic elimination. This process however can lead to the formation of isomers by the SMILES rearrangement.
Scheme 1
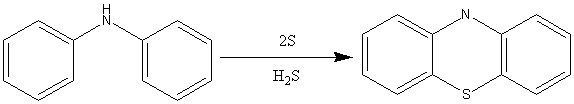
Scheme 2
More complex phenothiazines can be made by the method shown in scheme 3. This is the reductive cyclisation of o-nitrodiphenyl sulphides with tri-phenyl phosphate. In this multistep process the nitro group is deoxygenated to a nitrene (a), ipso attack n the other benzene ring then leads to the formation of a sprobetaine intermediate (b). The final two steps convert the five membered spirobetaine ring of the intermediate to a six membered ring. This process is analogous to a SMILES rearrangement.
Scheme 3

Spectral Data
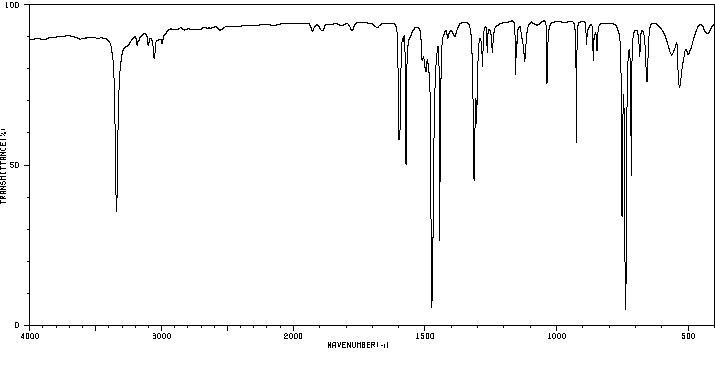
Infra-red Spectra [4]
With KBr Discs:
H1-NMR Spetrum [5]
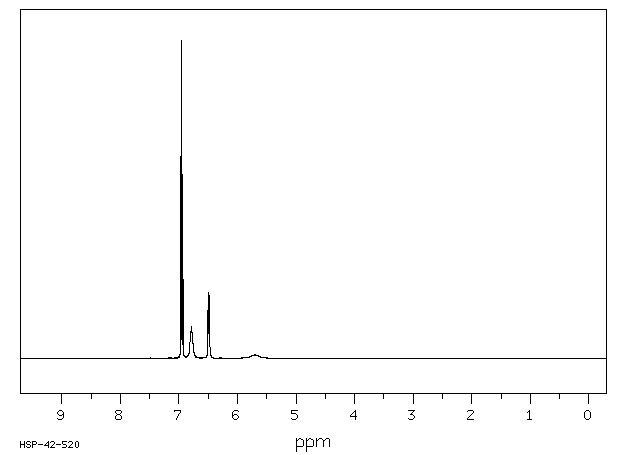
This is the H1NMR spectrum, run using CDCl3 solvent at 50oC. The peak assignment can be seen below:
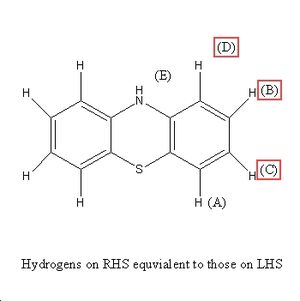
Shift 6.96ppm = A, Shift 6.95ppm = B, Shift 6.79ppm = C, Shift 6.49ppm=D and Shift 5.70ppm=E.
Mass Spectrum [6]
The molecular ion peak has a mass charge ratio of 199.0.
Applications
Pharmaceutical [7]
The phenothiazines are used as anti-emetics and anti-psychotics.
Antiemetics: These are drugs that relieve nausea and vommiting. Phenothiazines are commonly used in migrane and motion sickness medication, in their anti-emetic capacity. Phenothiazines are dopamine antagonists and act by blocking the nerve transmission at the receptor. They are more frequently used in preventing nausea associated with radiation sickness, neoplastic disease and vomitting caused by opiods and general anaesthetics. They are used only in severe motion sickness.
A commonly used anti-emetic phenothiazine is pro-choloroperazine, this is used for severe vomitting and vertigo. It is usually adminstered orally but can be administered as a deep intra-muscular injection. The trade name for pro-cholorperazine is Stemetil. The molecule displayed below is pro-chloroperazine.
Anti-psychotics:Broadly speaking there are three categories of phenothiazine derived anti-psychotic drug.
Category 1 includes: fluphenazine, perphenazine, prochlorperazine, and trifluoperazine. These drugs have more intense extrapyramidal side effects, but they have reduced sedative effects.
Category 2 includes: pericyazine and pipotiazine, these have more marked sedative and antimusaric effect. They do present fewer extrapyramidal side effects that category 1.
Category 3 includes: chlorpromazine, levomepromazine (methotrimeprazine), and promazine. These drugs have marked sediative effects, with reduced extrapyramidal and antimusaric.
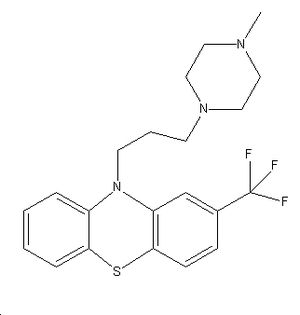
More detail on trifluoperazine can be seen below...
Trifluoperazine is a phenothiazine used in the treatment of mood disorders and schizophrenia. It usage in modern medicine has been slowly declining due to its highly pronounced extrapyramidal side-effects. These extrapyramidal effects include pancytopenia (reduction in blood cells and platelets), thrombocytopenia (reduction in platelets), hyperpyrexia(unusual and excessive elevation in body temperature) and anorexia. Below is an animation of and diagram of the structure of trifluoperazine.
Pentahelicene |
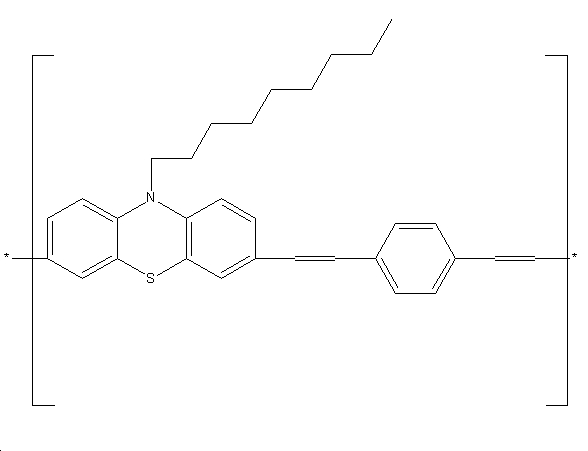
Light Emitting Diodes [8]
Phenothiazine derivatives such as poly(3,7-N-octyl phenothiozinyl terephthalylidene) (see below) are used as light emitting diodes. This particular diode emits a 100% pure yellow light of wavelength 575nm. There are many other examples of the phenothiazines being used as transistors and light emitting diodes.
Pesticides
Phenothiazine derivative pesticides work by supressing the insects nervous system . The attack then kills or permenantly disables the insect (or occasionally small animals)[9]. Interestingly some phenothiazines can be used in humans to reverse the effects of common rate poisions[10].
References
1. (a) Hromatka and et al.: 307,311 ;BRN 11497 ;BPR 37004-69-6.
(b)http://physchem.ox.ac.uk/msds/ (The Physical and Theoretical Chemistry Laboratory Oxford University, 01/11/2007)
2. Eicher, T and Hauptmann, S. The Chemistry of Heterocycles. Wiley-VCH. 2nd Edition, 2003.
3. SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 01/11/2007)
4. BNF.org: http://bnf.org/bnf/bnf (The British National Formulary, British Medical Journal, 03/11/2007)
5. Cao, H. H. (2007). "Undoped yellow-emitting organic light-emitting diodes from a phenothiazine-based derivative." Synthetic metals 157(10): 427.
6. Byrne, M. J. (1976). "HIGH-SPEED LIQUID-CHROMATOGRAPHIC DETERMINATION OF PHENOTHIAZINE IN COMMERCIAL PESTICIDE FORMULATIONS." Journal of the Association of Official Analytical Chemists 59(3): 693-695.
7. Nicholson, R.A. (1991) .Method of combating flupropadine poisoning using phenothiazines. United States Patent 5023073
(Jp806 15:18, 16 November 2007 (UTC))
- ↑ Hromatka and et al.: 307,311 ;BRN 11497 ;BPR 37004-69-6.
- ↑ http://physchem.ox.ac.uk/msds/ (The Physical and Theoretical Chemistry Laboratory Oxford University, 01/11/2007)
- ↑ Eicher, T and Hauptmann, S. The Chemistry of Heterocycles. Wiley-VCH. 2nd Edition, 2003.
- ↑ SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 01/11/2007)
- ↑ SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 01/11/2007)
- ↑ SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 01/11/2007)
- ↑ BNF.org: http://bnf.org/bnf/bnf (The British National Formulary, British Medical Journal, 03/11/2007)
- ↑ Cao, H. H. (2007). "Undoped yellow-emitting organic light-emitting diodes from a phenothiazine-based derivative." Synthetic metals 157(10): 427.
- ↑ Byrne, M. J. (1976). "HIGH-SPEED LIQUID-CHROMATOGRAPHIC DETERMINATION OF PHENOTHIAZINE IN COMMERCIAL PESTICIDE FORMULATIONS." Journal of the Association of Official Analytical Chemists 59(3): 693-695.
- ↑ Nicholson, R.A. (1991) .Method of combating flupropadine poisoning using phenothiazines. United States Patent 5023073