It07:Phenolphthalein
| It07:Phenolphthalein | |
|---|---|
| |
| General | |
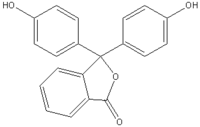
| Systematic name | 3,3-bis-(4-hydroxy-phenyl)-3H-isobenzofuran-1-one |
| Other names | Phenolphthalein |
| Molecular formula | C20H14O4 |
| SMILES | Oc1ccc(cc1)C3(OC(=O)c2ccccc23)
c4ccc(O)cc4 |
| Molar mass | 318.323 g mol-1 |
| Appearance | White, thick, crystalline powder |
| CAS number | [77-09-8] |
| Properties | |
| Density & phase | 1.277 g/cm³ at 32ºC |
| Solubility in water | 0.092 g/L (25°C) |
| Melting point | 536 K |
| Acidity (pKa) | 9.3 at 25ºC |
| Crystal structure | Orthorhombic |
| Hazards | |
| Main hazards | Harmful, Irritant, Suspected Carcinogen |
3D Structure
Overview
The Oxford English Dictionary defintion of Phenolphthalein describes it as a 'crystalline solid used as an acid-base indicator and medicinally as a laxative'[1]. This essentially reveals the two gifts this compound has given to the chemical and medicine worlds respectively.It did however not state it was white or pale yellow in colour and generally a thick powder. Typically, it also excluded the fact that phenolphthalein was originally synthesised by condensation of phthalic anhydride with phenol.
Uses
Of course it would be highly disrespectful to such an versatile substance, to ignore the intricacies of its varied uses; intricacies that can determine whether a solution will accept or donate protons, relieve the digestive system of grave discomfort, and, if you read on a little further, save you from serving a life sentence; if your a clumsy taco eater of course.
Laxative
It was actually 31 years later when it was realised that phenolphthalein could be used as a laxative. However it is not yet known in what instance, whether through painful experience or otherwise, Hungarian Professor Zlotan Vamossy discovered these attributes. It was much later in 1948 that S.Loewe of Cornell University Medical Collegem concluded that due to its pathetic solubility in water, our substance in question actually remained undissolved in the stomach only to be later be later dissolved by bile salts such as taurocholic acid and deoxycholic acid.3 Naturally a small proportion of this would be absorbed and released in urine, but it’s the remaining amount where the real action kicks in. Essentially, phenolphthalein resurrects the process of peristalsis in the large intestine, which during constipation is severely limited. It does this by stimulating the mucosa membrane and constricting the smooth muscles both of the large intestine wall. Some academics felt that this was due to the irritating effect of phenol, but Loewe in his article for J. Pharmacol. & Exper. Therap. quelled this idea.[2] Like all other stimulant laxatives, phenolphthalein affects the water and electrolyte transport balance which also helps push dormant faeces push through the colon. This combination of actions makes our friend phenolphthalein a particularly potent laxative, the effects of which can be felt only 6-8 hours after oral consumption[3]
Indicator
If that wasn’t enough, then phenolphthalein ingratiates itself into the fascinating world of titrations by also being a particularly efficient indicator. In a nutshell, it does this by being colourless when acidic and pink when alkaline. The advantage of this is that a solution with phenolphthalein will either be one or the other colour and immediate determination can then be done. Easy! Easy! This removes any ambiguity that may arise from using universal indicator because it may be a number of colours depending on the solutions very specific pH.
The reasoning is that phenolphthalein is a weak acid, which obviously wants to lose a proton to become an ion. Phenolphthalein is colourless while the ion is pink. Therefore in acidic solution it cannot lose a proton to save its life and stays colourless, while in basic conditions it can, will form the ion, and turn pink. [4] As a practical point, at a extremely high pH, the solution can turn colourless agin , while at a extremely low pH it can turn orange.
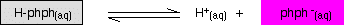
Dangers
However the phenolphthalein constipation relief love story has hit a rocky patch of late, very rocky indeed. Studies performed In Los Angeles and North Carolina at the turn of the 1980’s concluded that rodents left open to phenolphthalein had developed colon cancer. Despite the fact that such an instance would need prolonged usage, phenolphthalein containing laxatives were soon brought off the market and in some countries were banned. However its use as an indicator is still in practice as prolonged usage is not needed.[5]
One danger that has definitive realistic effects upon consumption, is the cathartic nature of phenolphthalein. As explained earlier, a portion of the substance is absorbed by the body, of which a certain amount is re-excreted back into the bile. As some phenolphthalein is left over after the initial action, defecation ensues at a faster rate producing a cathartic effect. After the desired bowel movement is achieved, the irony is that constipation may occur and so a vicious cycle ensues where the patient feels the need for more of it to combat the resultant side effect, giving a pseudo-addictive symptom. It is because of this prolonged use that the carcinogenic property of phenolphthalein becomes more of a threat. Now we can see that phenolphthalein is not so wondrous after all.[6]
Controversy
Controversy I hear you cry. How can such a dynamic chemical be at the centre of controversy? Well, it has, and not just once but twice. The first instance resulted from the study aforementioned regarding phenolphthalein’s suspected carcinogenetic properties. When realised, the producers of America’s two biggest selling laxatives, both containing phenolphthalein, ensued in a dog-eat dog corporate battle to once and for all colonise the market. Schering-Plough decided to reformulate their winning laxative, Correctol, with bisacodyl rather than phenolphthalein, while rival Novartis decided to whether the storm by staying put with their phenolphthalein containing ‘Ex-Lax’ but, attempting to land the corporate sucker punch, also ran a substantial advertising campaign questioning the validity, safety and efficiency of Schering-Plough’s new drug. Evidently, in a country where pharmacy and medicine means big money, this very public conglomerate slagging match did nothing but shoot those in question in the foot. The customers, instead of steering clear of particular brands warned in respective advertisement campaigns, ironically steered clear of the whole market.[7]

Our friend was also involved in the biggest court case of the 1990’s, Yes that’s right, phenolphthalein even had a cameo role in the unforgettable OJ Simpson trail. A stain, presumed to be the victim’s blood, was found on a piece of Mr. Simpson's clothing, and phenolphthalein was used to test if it was actually blood, known as the Kastle-Meyer test. It works like this: reduced phenolphthalein can be oxidised back to phenolphthalein by oxygen, and haemoglobin in the blood can form this oxygen from hydrogen peroxide, also found in the blood. If the reformed phenolphthalein is then treated with a base, and turns pink, then the suspected stain must be blood, because if it wasn’t, the reduced phenolphthalein wouldn’t have been oxidised, and the resultant product wouldn’t have turned pink! Incidentally, this test was carried out and a pink colour was given when the formed phenolphthalein was treated with base, therefore suggesting it was blood on Mr Simpson’s clothing. However in the slippery road of a judicial court room, the defence stated that, of all things, taco sauce also contains such chemicals to oxidise the reduced phenolphthalein, giving no conclusion as to what the stain actually was, and so ignored by the jury. The rest, as they say, is history.[8]
IR Spectrum
Mass Spectrum
References
- ↑ Oxford Dictionary http://www.askoxford.com/concise_oed/phenolphthalein?view=uk Date Accessed:30/11/07
- ↑ Loewe, S. : Studies on the laxative activity of triphenylmethane derivatives: Relationship between structure and activity of phenolphthalein congenes. J. PharmacoI. & Exper. Therap. vol 94: 288, 1948.
- ↑ Ravdin P.M., van Beurden M., Jordan V.C., Estrogenic effects of phenolphthalein on human breast cancer cellsin vitro, Breast Cancer Research and Treatment, 9: 2: pg 151-4, 2005
- ↑ http://www.chemguide.co.uk/physical/acidbaseeqia/indicators.html Date Accessed: 3/12/07
- ↑ Longnecker M.P., Sandler D.P., Haile, R.W., Sandler R.S., Phenolphthalein-containing Laxative Use in Relation to Adenomatous Colorectal Polyps in Three Studies, Environmental Health Perspectives, 105: 11, 1997
- ↑ Mechling R.S., The pharmacology of cathartics, Diseases of the Colon & Rectum, 1: 5. 1958
- ↑ Eder R., Resolving the phenolphthalein flap - active ingredient's potential health risks affect marketing strategy of laxative manufacturers - Chain Pharmacy, Drug Store News, 14/7/97, http://66.102.9.104/search?q=cache:E8hkwz7ol1cJ:findarticles.com/p/articles/mi_m3374/is_n11_v19/ai_19597698+phenolphthalein+discovery+as+laxative&hl=en&ct=clnk&cd=1&gl=uk Date Accessed: 3/12/07
- ↑ Schwartz J. Dr, The Genie in the Bottle, Published 2001, ECW Press, pg 158-160, http://books.google.com/books?id=hkcnypiDj7QC&pg=PA158&lpg=PA158&dq=phenolphthalein+1880&source=web&ots=Yjtzf4zyQv&sig=TLw3VQLXffeMu2z3T7o77g9irhU#PPA158,M1