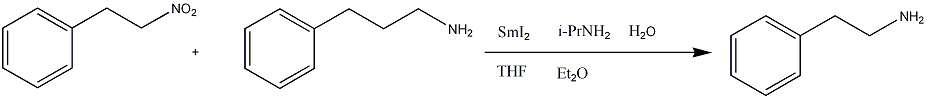It07:Phenethylamine
Introduction
Phenethylamine is a colourless-slightly yellow liquid with a fishy odour that is characteristic of amines. It is an alkaloid that belongs to a class of molecules related to adrenalin and occurs naturally in the human body due to its biosynthesis from the amino acid phenylalanine, being broken down in the body by the enzyme monoamine oxidase B. Phenethylamine derivatives are present in some citrus fruits and the compound itself is found in chocolate. The occurrence of a migraine in some people after the consumption of chocolate has been associated with a build up of phenethylamine in the body due to a lack of the enzyme required to metabolise it.
| It07:Phenethylamine | |||
|---|---|---|---|
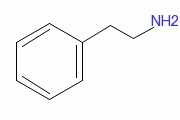
| |||
| General | |||
| Systematic name | Phenethylamine | ||
| Other names | Phenaethylamin, 2-Phenylethylamine, benzeneethanamine, beta-Phenylethylamine | ||
| Molecular formula | C8H11N | ||
| SMILES | NCCc1ccccc1 | ||
| Molar mass | 121.18g/mol | ||
| Appearance | Colourless/yellow liquid | ||
| CAS number | 60-04-0 | ||
| Properties | |||
| Density & phase | 0.964g/cm³ | ||
| Melting point | -60°C | ||
| Boiling point | 197-200°C | ||
| Chiral rotation [α]D | 1.533D | ||
| Hazards | |||
| MSDS | External MSDS | ||
| Flash point | 90°C | ||
| R/S statement | R: 22,34 S: 26,36,37,39,45 | ||
| RTECS number | SG8750000 | ||
Biochemical EffectsThe process of redox cycling of phenethylamine and its derivatives releases oxygen free radicals. These free radicals are harmful inside the body; they cause oxidative damage, in a similar way to amphetamine abuse. Furthermore, they have been associated with emphysema, cancer, heart disease and stroke. The cells of the nervous system and brain are the most susceptible to damage by the production of the free radicals. If phenethylamines are taken as part of a medical treatment then substances that remove them must be taken in conjunction to prevent harmful side effects as a result of cell damage. Such counter substances include antioxidants and the appropriate vitamin and mineral supplements[1]. Synthesis[2].
SafetyPhenethylamine is corrosive and can cause severe burns on contact. Ingestion cause burns to the mouth and throat nad can lead to vomiting and diarrhoea. It is flammable and above it’s flash point (90oC), it can form explosive vapour mixtures[3]. Combustion can lead to the production of toxic/irritant compounds such as carbon monoxide and nitrogen oxides.
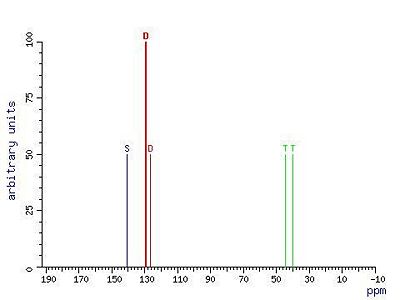
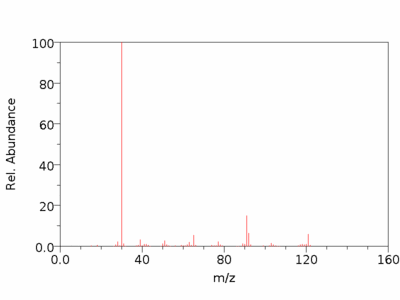
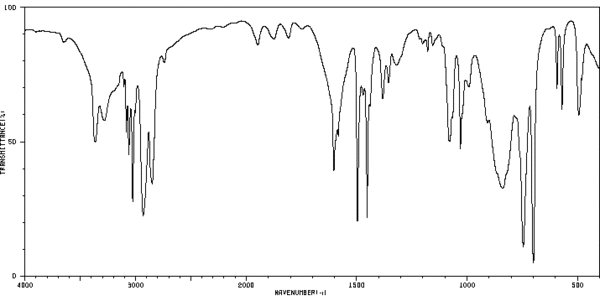
SpectraC-13 NMR Spectrum[4]Mass Spectrum[5]IR Spectrum[6]References
|
|||