It07:Ozone
Ozone
| It07:Ozone | |
|---|---|
| |
| General | |
| Systematic name | Ozone |
| Other names | Trioxygen |
| Molecular formula | O3 |
| Molar mass | 47.998 g·mol−1 |
| Appearance | Gas (293K) |
| CAS number | 10028-15-6] |
| Properties | |
| Density & phase | 2.144 g·L−1 (0 °C), gas |
| Solubility in water | 0.105 g·100mL−1 (0 °C) |
| Melting point | 80.7 K, −192.5 °C |
| Boiling point | 161.3 K, −111.9 °C |
About
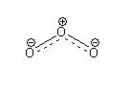
Ozone is a well known molecule consisting of three oxygen atoms bonded in a bent arrangement, with a positive charge on the central atom and a partial distrubuted negative charge across the terminal oxygen atoms and bonds. It is found in the upper atmosphere and protects the earth's surface from intense UV radiation, although ozone itself is a pollutant. Also ozone is a by-product produced from photocopying machines. This is why it is recommended for photocopying from a photocopying machine to be done in a well ventilated area.
Uses
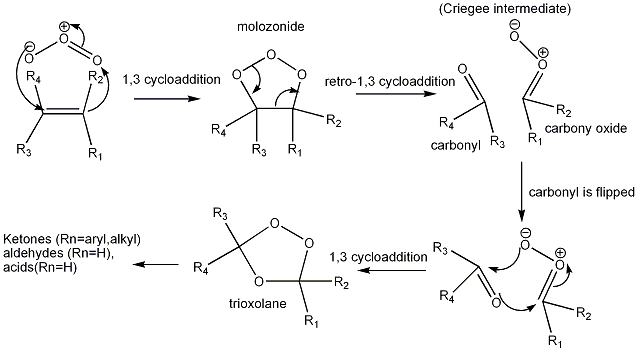
Ozone can be used in organic synthesis in a process called Ozonolysis. This synthesis takes a alkene to molozonide and can be further converted to two aldehydes. RC=CR' get converted to RC=O and R'C=O. The mechanism involves the formation of a primary 5 membered ring structure followed by a rearrangement of the ring, then on addition of say PPh3 the ring is attacked and the two R aldehydes form.
Structure of ozone
2D structure
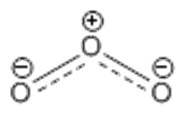
Ozone is a triatomic bent molecule, with C2V symmetry. It can be drawn as a central sp2 hybridised central oxygen atom, bound to two oxygen atoms with one double bond and one single bond. The signally bonded oxygen carries a single formal negative charge whilst the central oxygen carries a single formal positive charge. Resonance structures can be drawn by swapping the double and single bonds over, both resonance structures contribute equally to the overall structure. In reality, ozone has a delocalised structure where the central oxygen carries a formal positive charge and the other two oxygens have half negative charges. Both bond lengths are equal at 1.28Å.
3D structure
Methamphetamine |
Ozone reactions
Mostly ozone reactions occur in the atomsphere. It acts as an unstable reactant in the sunlight. These reactions are described in high ozone layer (stratosphere) and low ozone layer (tropospheric ozone).
High ozone layer:
Stratosphere layer is 10km and 50 km above the earth surface.
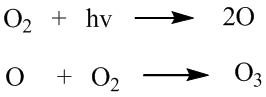
It then filters out the UV lights with shorter wavelength (from 270nm to 440nm),which is harmful to mamy things. Ozone in the high layer is alomost produced by oxygens and UV light. The above reaction is the formation of ozone. Ozone is a 3-atoms molecule, which is in higher energy than the 2-atoms oxygens. In order to create this higher energy molecule, we need a large amount of energy to do this. This energy is then provided by the UV photons with high energy. The photon reacts with the oxygen molecule to give to 2 oxygen atoms. After the formation of 2 oxygen atoms,one of them then react with oxygen to give ozone.
Low ozone layer:
tropospheric ozone layer is about 10km and 18km above the earth surface. The ozone within this region is reacting with chemical compounds produced mainly by human. The occuring of these depleting reactions are due to the strong oxidising property of ozone and the presence of the catalytes. Ozone depleting reactins can be readily catalysed by bromine or chlorine. These bromine and chlorine are contained in many oganic compounds produced by human, such as brominated halons or chlorofluorocarbons (CFC's).
For the CFC's:
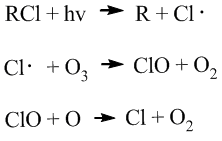
The CFC is photolyzed by UV light that breaks the weakest bond, usually a C-Cl bond. The Cl radical is formed and then reacts with ozone.
Brominated halons follow the similar above reation, but its C-Br bond is broken by the UV light.
Oxdising reaction:
Since ozone can act as a strong oxiding agent, it can react the following reaction.

References
http://www.princeton.edu/~chm333/2002/spring/Ozone/chemistry_formation.htm
