It07:Oseltamivir Phosphate
Oseltamivir Phosphate (Tamiflu)
| It07:Oseltamivir Phosphate | |
|---|---|
| |
| General | |
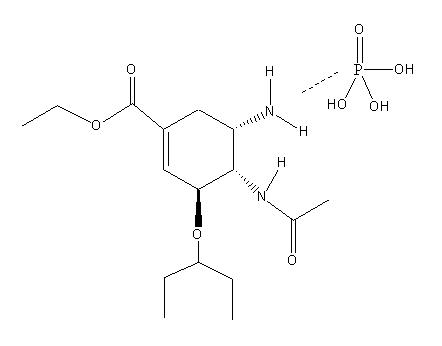
| Systematic name | (3R,4R,5S)-4-acetylamino-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester phosphate(1:1) |
| Other names | Tamiflu |
| Molecular formula | C16H31N2O8P |
| Molar mass | 410.4 |
| Appearance | White Solid |
| CAS number | 204255-11-8 |
| Properties | |
| Melting point | 189-191 K |
| Chiral rotation [α]D | -30.5° (0.480g/100ml H2O 24C) |
3D Structure
Uses
- Oseltamivir Phosphate, or Tamiflu, is used in medicine for the treatment of influenza types A and B. It has recently been in the media spotlight due to claims that it can treat the H5N1 virus, Avain Influenza, or more commonly Bird Flu. The molecule works by using the active Oseltamivir base, having the effect of neuraminidase inhibition. It acts as a transition-state analogue inhibitor of influenza neuraminidase, it prevents the progeny virions of teh influenza from escaping the infected cells.
- Typical dosage is 75mg twice a day for humans over the age of one. The treatment must cimmence within 48 hours of flu-like symptoms occuring for maximum effect of the inhibitor.
Synthesis
Commercial Synthesis (Hoffman-La Roche)
The current patent holder is Hoffman-La Roche who use the Karpf / Trussardi synthesis, this synthesis poses health risks as it involves handling two azides in the process.
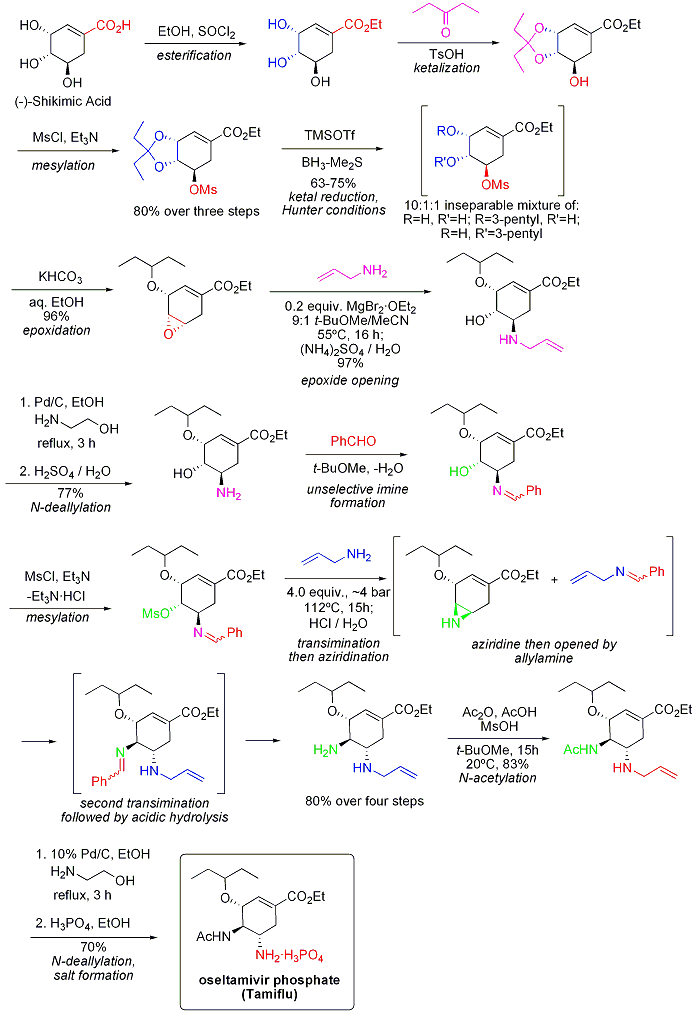 [1]
[1]
Other Synethesis Routes
There are three more major synthesis routes that are used in small scale production, the Corey synthesis[2] this method does not use the starting acid and the end product is a BOC derivative which can then be converted to the phosphate. There is also the Shibasaki Synthesis [3] [4]which again bypasses the starting acid used in the commercial synthesis, the difference to the Corey synthesis is that it is a two stage synthesis that ends up with the phosphate. Finally there is the Fukuyama synthesis[5] which starts with an asymmetric Diels-Alder reaction instead of the acid.
See Also
References
- ↑ New, Azide-Free Transformation of Epoxides into 1,2-Diamino Compounds: Synthesis of the Anti-Influenza Neuraminidase Inhibitor Oseltamivir Phosphate (Tamiflu) Martin Karpf and René Trussardi J. Org. Chem.; 2001; 66(6) pp 2044 - 2051; (Article) DOI:10.1021/jo005702l .
- ↑ A Short Enantioselective Pathway for the Synthesis of the Anti-Influenza Neuramidase Inhibitor Oseltamivir from 1,3-Butadiene and Acrylic Acid Ying-Yeung Yeung, Sungwoo Hong, and E. J. Corey J. Am. Chem. Soc.; 2006; 128(19) pp 6310 - 6311; (Communication) DOI:10.1021/ja0616433
- ↑ De Novo Synthesis of Tamiflu via a Catalytic Asymmetric Ring-Opening of meso-Aziridines with TMSN3 Yuhei Fukuta, Tsuyoshi Mita, Nobuhisa Fukuda, Motomu Kanai, and Masakatsu Shibasaki J. Am. Chem. Soc.; 2006; 128(19) pp 6312 - 6313; DOI:10.1021/ja061696k
- ↑ Second Generation Catalytic Asymmetric Synthesis of Tamiflu: Allylic Substitution Route Tsuyoshi Mita, Nobuhisa Fukuda, Francesc X. Roca, Motomu Kanai, and Masakatsu Shibasaki Org. Lett.; 2007; 9(2) pp 259 - 262; (Letter) DOI:10.1021/ol062663c
- ↑ A Practical Synthesis of (-)-Oseltamivir Nobuhiro Satoh, Takahiro Akiba, Satoshi Yokoshima, Tohru Fukuyama Angew. Chem. Int. Ed. 2007, 46, 5734 –5736 DOI:10.1002/anie.200701754 10.1002/anie.200701754
