It07:Octanitrocubane
| It07:Octanitrocubane | |
|---|---|
| |
| General | |
| Systematic name | Octanitrocubane |
| Other names | {{{OtherNames}}} |
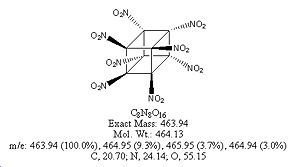
| Molecular formula | C8N8O16 |
| SMILES | O=N(=O)C12C3(N(=O)=O)C4(N(=O)=O)C1(N(=O)=O)
C5(N(=O)=O)C2(N(=O)=O)C3(N(=O)=O)C45N(=O)=O |
| Molar mass | 464.1296 g/mol |
| Appearance | {{{Appearance}}} |
| Space group | O62h |
| CAS number | {{{CASNo}}} |
| Properties | |
| Density & phase | 2 g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | {{{Mp}}} K |
| Boiling point | {{{Bp}}} K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | {{{Hazards}}} |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
3D structure and crystal unit cell
1 3D structure of octanitrocubane.
Right click on molecule to explore
3D crystal unit cell structure.
Right click on molecule to explore
Cell parameter values in Å
About Octanitrocubane
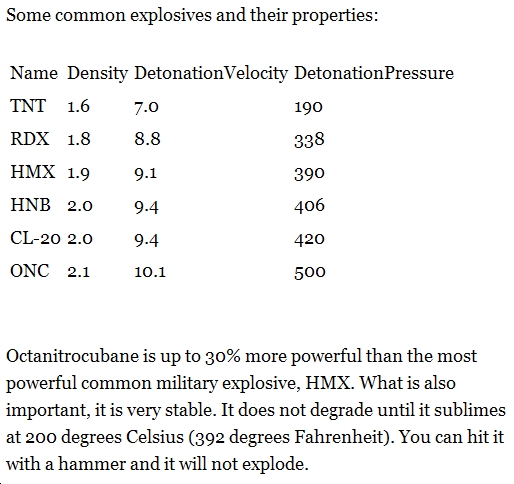
Octanitrocubane is the most potent explosive to date and is kinetically inert 2. It has an R.E. factor of about 2.7 which is nearly 3 times as much as TNT! The extreme explosiveness of the molecule is due to 2 things. The first is due to the expansive breakdown of the molecule into 8CO2 + 4N2. This breakdown is highly exothermic and provides a significant driving force. The second is due to the high bond strain in cubane. An advantage of using octanitrocubane as an explosive is that the decomposition products are cosidered non toxic and it requires no additional oxygen to decompose as well as producing no water vapour. A disadvantage is that because of the complexity and instability of the molecule, it cannot be manufactured in large quanities. Octanitrocubane is the most dense explosive to date. The denser the explosive, the higher its detonation velocity (greater power).
3
Synthesis
Octanitrocubane is a very difficult compound to make due to its complex geometry. To make cubane itself, a 17 step process is needed and to create octanitrocubane itself, a total of 40 synthetic steps are needed! This also explains why octanitrocubane is not made in large quantities. To get an idea of how complicated its synthesis is, the following webpage shows how to synthesise tetranitrocubane in a 17 step process: http://www.chemsoc.org/exemplarchem/entries/2004/icl_Wang/nitro2.html
References
- 1)Mao-Xi Zhang, P.E Eaton, R Gilardi, Angew Chem Int.ed, 2000, vol 39, pg 401
- 2)http://sci-toys.com/attention/2006/04/octanitrocubane-most-powerful.html
- 3)http://sci-toys.com/attention/2006/04/octanitrocubane-most-powerful.html