It07:Nicotine
Nicotine
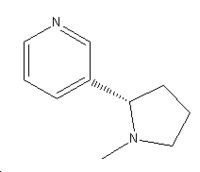
A three dimesional representation of nicotine
Nicotine |
A three dimensional representation of the crystal structure of a unit cell of nicotine iodide
nicotine iodide |
| It07:Nicotine | |
|---|---|
| |
| General | |
| Systematic name | 3-[(2S)-1-methyl-2-pyrrolidinyl]Pyridine |
| Other names | Nicotine, L-Nicotine, .beta.-Pyridyl-.alpha.-N-methylpyrrolidine |
| Molecular formula | C10H14N2 |
| SMILES | CN1CCC[S@H]1[C@]2=CC=CN=C2 |
| Molar mass | 162.23 |
| Appearance | {{{Appearance}}} |
| CAS number | 54-11-5 |
| Properties | |
| Density & phase | 1.0068 g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | 496-497 K |
| Boiling point | 521 K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Dynamic Viscosity at 20°C | 0.04536 gcm-1s-1[1] |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | {{{Hazards}}} |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Nicotine is an alkaloid. The compound is famously known for being contained in the tabacco products. This compound is also contained in some of the vegetables such as totato, potato and green pepper. This can be proven by carrying out GLC, TLC or CC-mass spectrometry. However the amount of nicotine contained in those vegetables is very small compare to the amount in tabacco product. In those vegitables, upto about 23ppm of nicotine is present. On the other hand, a typical pack of cigarette contains around 1mg of nicotine.[2]
Biosynthesis of Nicotine in Tobacco plant
[3]
Nicotine is synthesized from a protein chain called polyamine putrescine at the root of tabacco plant. The nicotine would then be transferred to the shoot of the plant and stored. Polyamine putrescine can be synthesised from ornithine with a presence of ornithine decarboxylate as a catalyst. On this pathway, CO2 is produced as a by-product. Another pathway is to react arginine in a presence of arginine dacarboxylate as a catalyst via forming agmatine. This pathway has three stages of reaction in order to get a desired product. The pathway is shown below.
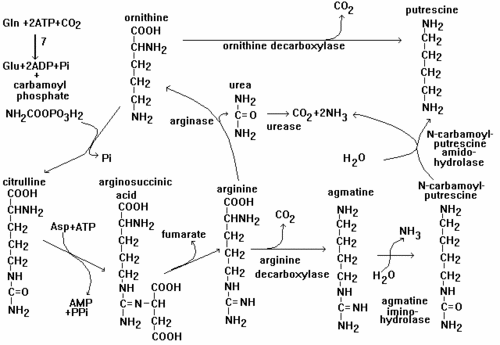 [4]
After the formation of polyamine purtrescine, the product is deaminated with putrescine N-methyltransferase enzyme (PMT) to form N-methylputrescine. Then Diamine oxdase(DAO) reacts with N-methylputrescine to form methyl pyrrolinium cation. This cation part will form the pyrrolidine part of the final molecule. Pyridine part of nicotine is formed in following. Aspartate molecule undergoes the NAD biosynthesis pathway to form 3-pyridine carboxylic acid (nicotinic acid). At this moment it is not know that either 3-pyrodine acid or its metabolised derivative react with methyl pyrrolinium cation to form nicotine.
[4]
After the formation of polyamine purtrescine, the product is deaminated with putrescine N-methyltransferase enzyme (PMT) to form N-methylputrescine. Then Diamine oxdase(DAO) reacts with N-methylputrescine to form methyl pyrrolinium cation. This cation part will form the pyrrolidine part of the final molecule. Pyridine part of nicotine is formed in following. Aspartate molecule undergoes the NAD biosynthesis pathway to form 3-pyridine carboxylic acid (nicotinic acid). At this moment it is not know that either 3-pyrodine acid or its metabolised derivative react with methyl pyrrolinium cation to form nicotine.
 [5]
A diagram of biosynthetic pathway
[5]
A diagram of biosynthetic pathway
Transportation process and defence mechanism involving nicotine in nicotine plant
Nicotine is biosynthesised in the root of the tobacco plant. After the production, nicotine is transferred to xylem which transports nicotine up to aerial parts of the plant such as leaf and lateral buds. Vacuoles in these parts of the plant accumulate the nicotine. Nicotine is produced as a defence mechanism against herbivores. There is evidence which suggests the damage on the plant effects the amount of nicotine produced and accumulated in tobacco plant. When the plant is damaged by insect herbivores, the plant increases concentration of Jasmonates (JA) which is a group of plant hormone. Jasmonic acid in JA acts on the enzymes to producing more nicotine. Hence more nicotine would be accumulated in vacuoles. This is experimentally proved. However, the concentration rise depends on the position of the damage. Puncturing the leaves has less effect on concentration of nicotine relative to removal of shoot apex. Tobacco plant without the damages accumulates 0.1-1% of its dry mass. However, damaging the plant could increase the concentration up to around 4% of its dry mass.
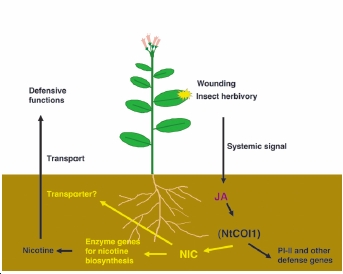 [6]
[6]
Diagram of nicotine production process
Nicotine in the body
Entry and lifetime
Nicotine can get into the body in various ways, the main channels into the body include:
- Skin
- Membranes (including the lining of the lungs and gums)
The diffusion into the body and hence time to get to the brain is quickest through the lungs which has an extremely high diffusion rate due to: Large surface area, moist lining, extremely thin lining and a good blood supply.
Once in the body nicotine is broken down mainly in the liver by conversion into cotinine through an enzyme pathway. It can also be metabolised in the lungs again to cotinine as well as nicotine oxide which is then excreted, along with remaining nicotine filtered in the kidneys, in the form of urine. These processes amount to the nicotine being reduced in amount by 97% in just 360 minutes after smoking the cigarette. Some people do have a natural genetic defect however which means the enzyme that breaks down nicotine is not as effective as it could be as so the nicotine remains in the blood a lot longer and so reduces the need for a nicotine "top-up" during the day. [7]
Effects whilst in the body
Whist nicotine is in the body it has many immediate effects. It has a biphasic nature in which it can either have a positive or negative effect on the general mood of the consumer. The way in which this effect swings is dependant on how much and how often nicotine is being given to the body.[8]
Nicotine also binds to acetylcholine receptors in the autonomic nervous system. This causes the change in mood and gives it its addictive properties, as the synaptic mebrane becomes less sensitive to both nicotine and acetylcholine after use of nicotine. This is shown more here: https://www.ch.ic.ac.uk/wiki/index.php/Acetylcholine#Function_Mechanism
When nicotine enters the body it causes a sudden release of adrenaline. This adrenaline has the effect of increasing blood flow rate as well as decreasing the size of the arteries. This has an overall effect of increasing blood pressure in the smoker. Adrenaline also lowers the amount of the hormone insulin in the blood which causes to some extent a hypoglycemic effect. This is thought to be a reason for a suppression in appetite prevalent in the smoker because the brain can detect the large amount of glucose and then slows down release of hormones telling the body it needs food.
Also intake of nicotine can result in an increase in LDL(low density lipoproteins) and decrease in HDL (high density lipoproteins), shown by experimental evidence. [9]
- Control: LDL= 140 mg/dl HDL 48.8 mg/dl
- Nicotine: LDL= 168 mg/dl HDL 27.8 mg/dl
This effect can in turn can lead to an elevation in concentration of triglycerides and cholesterol in the body and also an to an increase in plaques in the arteries. This leads to an increased chance of atherosclerosis, myocardial infarction or cerebral infarction.
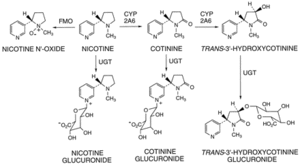
Cytochrome P450 2A6 (CYP2A6) |
The above protein (Cytochrome P450 2A6) is one of the most important enzymes in human nicotine metabolism. website [12]
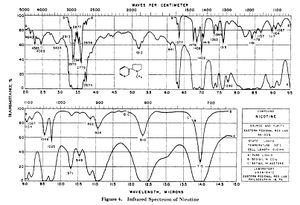
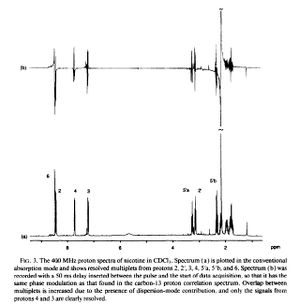
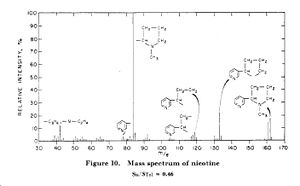
Reference
- ↑ Journal:Z.Phys.Chem.Stoechiom.Verwandtschaftsl, Page37, Volume 68, 1909
- ↑ Journal of Food Science, Volume 53 Issue 5 Page 1572-1573, September 1988,"Abstract Detection of Nicotine in Foods and Plant Materials", Shun J. Sheen, DOI:10.1111/j.1365-2621.1988.tb09328.x
- ↑ Journal of Experimental Botany Page2899-2907 Volume57 Number 11 August 2006, "Nicotine synthesis in Nicotiana tabacum L. induced by mechanical wounding is regulated by auxin", Qiumei Shi, Chunjian Li, and Fusuo Zhang DOI:10.1093/jxb/erl051
- ↑ http://www.hort.purdue.edu/rhodcv/hort640c/polyam/po00001.htm
- ↑ Plant Biotechnology 22, 389–392 (2005) "Molecular regulation of nicotine biosynthesis", Akira Katoh, Hiroyuki Ohki, Koji Inai, Takashi Hashimoto http://db1.wdc-jp.com/pdf_store/jspcmb/pdf/pb22_5/22_389.pdf
- ↑ Plant Biotechnology 22, 389–392 (2005) "Molecular regulation of nicotine biosynthesis", Akira Katoh, Hiroyuki Ohki, Koji Inai, Takashi Hashimoto http://db1.wdc-jp.com/pdf_store/jspcmb/pdf/pb22_5/22_389.pdf
- ↑ Howstuff works website: http://health.howstuffworks.com/nicotine2.htm
- ↑ J. Foulds, J. A. Stapleton, N. Bell, J. Swettenham, M. J. Jarvis and M. A. H. Russell, Drug and Alcohol Dependence, 1997, 44, 105-115. DOI:10.1016/S0376-8716(96)01327-0
- ↑ T. Strohschneider, M. Oberhoff, H. Hanke, A. Hannekum and K. R. Karsch, Journal of Molecular Medicine, 1994, 72, 908-912.DOI:10.1007/BF00190750
- ↑ Wikimedia: http://commons.wikimedia.org/wiki/Image:Atherosclerosis,_aorta,_gross_pathology_PHIL_846_lores.jpg
- ↑ N. L. Benowitz, E. J. Perez-Stable, I. Fong, G. Modin, B. Herrera and P. Jacob, III, J Pharmacol Exp Ther, 1999, 291, 1196-1203.
- ↑ Summary of Nicotine addiction: http://www.cogsci.ecs.soton.ac.uk/cgi/psyc/ptopic?topic=nicotine-addiction
- ↑ J. J. Titman, D. Neuhaus and J. Keeler, Journal of Magnetic Resonance (1969), 1989, 85, 111-131.DOI:10.1016/0022-2364(89)90324-7
- ↑ Anal. Chem.; 1956; 28(3) pp 306 - 316, "Mass Spectrometric Analysis Broad Applicability to Chemical Research", F. W. McLafferty, DOI:10.1021/ac60111a005
