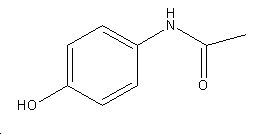
It07:N-(4-hydroxyphenyl)ethanamide
N-(4-hydroxyphenyl)ethanamide
| N-(4-hydroxyphenyl)ethanamide | |
|---|---|
| |
| IUPAC Systematic name | |
| N-(4-hydroxyphenyl)ethanamide | |
| Other name | |
| Paracetamol, Acitaminophen, Acetanilide, 4'-hydroxy-; p-(Acetylamino)phenol; p-Acetamidophenol; p-Hydroxyacetanilide etc | |
| Indentifiers | |
| ATC Code | N02BE01 |
| CAS number | 103-90-2 |
| PubChem (CID) | 1983 |
| SMILES | OC1=CC=C(NC(C)=O)C=C1 |
| Chemical Data | |
| Molecular formula | C8H9NO2 |
| Molar mass | 151.6 g/mol |
| Pharmacokinetic Data | |
| Bioavailability | Almost 100% |
| Protein Binding | 90 - 95% Hepatic |
| Metabolism | {{{Metabolism}}} |
| Half life | 1 - 4 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) B(US) safe |
| Legal status | Unscheduled(AU) GSL(UK) OTC(US) |
| Routes | Oral, rectal, intravenous |
Introduction
N-(4-hydroxyphenyl)ethanamide, or paracetamol, is the active metabolite of phenacetin, which was originally isolated from coal tar. It is currently one of the most popular over-the-counter drugs, as it is an effective analgesic and antipyretic and can be used as a safer alternative to aspirin. The most common use of paracetamol is for the management of low level pain and relief from fever. As such, it is a major ingredient in over-the-counter cold and flu remedies such as Beechams[1] and Lemsip[2] and is available in many different forms including syrup, drops, soluble tablets and normal tablets. Other major paracetamol contanining products include Panadol (a brand of GlaxoSmithKline) and Tylenol. Paracetamol can also be used for more sever pain, as it reduces the amount of anti-inflammatory drugs that are needed, thereby lessening the side-effects felt. Unlike aspirin, however, it does not work as an anti-inflammatory.
Recommended Dosage
- 3 months – 1 year : 60 – 120 mg
- 1 year – 3 years : 120 – 240 mg
- 3 years – 12 years : 240 – 360 mg
- Over 12 years : 360 – 500 mg[3]
- Adults : 500 - 1000 mg
Paracetamol can be taken every 4 - 6 hours, but should not be taken more than 4 times in 24 hours.
History
N-(4-hydroxyphenyl)ethanamide was first synthesised in 1893 as an alternative to an earlier analgesic, phenacetin. The first clinical trials into the drug took place at the Bayer Company and were supervised by an eminent chemist of the time, Joesph von Mering. N-(4-hydroxyphenyl)ethanamide proved to be a good analgesic and antipyretic but had one major drawback of causing methaemoglobinaemia (a condition in which the Fe(II) in haemoglobin is oxidised to Fe(III), hindering oxygen transport around the body and leading to cyanosis). This side-effect was not fatal, but caused unpleasant symptoms such as darkening skin and blue lips. It is now thought that these side-effects were being caused by some other toxic impurity but at the time they were considered enough for Mering to suspend the trials. Paracetamol was not re-investigated until 500 years later amid growing concerns about the safety of the long-term use of aspirin.
Paracetamol was first sold commercially by McNeil Laborotories as a relief for pain and fever in children and it was marketed under the name Tylenol Children's Elixir. This was followed by sale in the UK in 1956 of 500 mg tablets by the name of Panadol (produced by Frederick Stearns & Co), a product that was at first only available by prescription.
The US patent of the original drug has long been expired, but variations of paracetamol are still patented in the US in 2007. These include patents for "fast release paracetamol tablets", "Pharmaceutical composition containing sympathomimetic amine salt and co-distillable additive" and "Acetaminophen formulation for joint pain relief".
Limitations
Although paracetamol is generally considered to be a relatively safe alternative to aspirin it does have limitations. If taken in overdose it can cause serious liver damage and even death. Because of this and the fact that it is so readily available it is often used by people trying to commit suicide. This liver damage is brought about by the way in which paracetamol is broken down by the body.
Synthesis
- Start with phenol and add nitric acid. (This nitrates the othro- and para- positions (relative to the hydroxyl group)
- Since only the 1,4 product is useful this is extracted by steam distillation
- The nitro group of the 1,4 product is reduced to an amino group
- The amino group is then acelyated
This is a simple synthesis as paracetamol does not have enantiomers so the usual synthesis problem of stereospecificity does not apply.
Pharamacological Activity
The ways in which paracetamol acts as an analgesic and and antipyretic were completely unknown until 1971, and are still relatively so. It is thought that the drug inhibits cyclo-oxygenase(COX), an enzyme which is involved in converting phospholipids into prostoglandins and thromboxanes (these increase blood flow and cause other changes that lead to inflammation and hence pain). Aspirin and other anti-inflammatory drugs perform this action at the site of pain, whereas in a clinical trial (with dogs) evidence was produced that suggested paracetamol acted specifically in the brain. This explains its action as an analgesic. However, the trials (conducted by Sir John Vane) used high concentrations of paracetamol. Low concentrations have been shown to have little effect on COX in the human brain. Even more recently, this theory has been given support.
Spectral Data
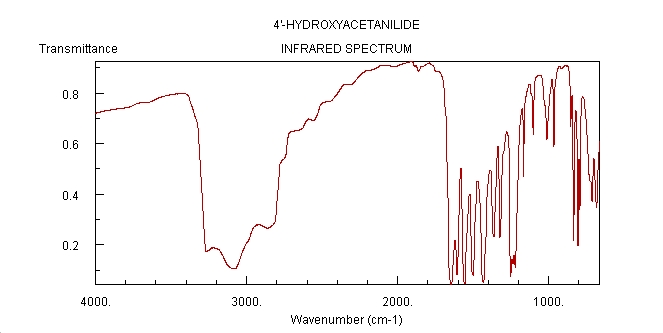
IR Spectrum
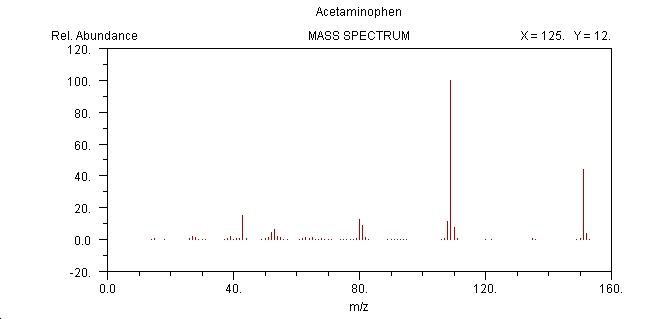
Mass Spectrum
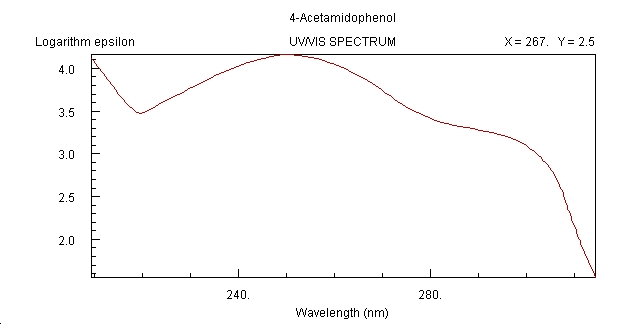
UV/Vis Spectrum
References
- http://www.beechamsfightback.co.uk/
- http://www.lemsip.com/max_lemon.php
- http://www.sheffieldchildrens.nhs.uk/patients/resources/039_paracetamol.pdf
- http://www.rsc.org/Education/EiC/issues/2005July/painrelief.asp
- IR Spectrum http://webbook.nist.gov/cgi/cbook.cgi?ID=C103902&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC
- Mass Spectrum http://webbook.nist.gov/cgi/cbook.cgi?ID=C103902&Units=SI&Mask=200#Mass-Spec
- UV/Vis Spectrum http://webbook.nist.gov/cgi/cbook.cgi?ID=C103902&Units=SI&Mask=400#UV-Vis-Spec