It07:Myristicin
Myristicin
Myristicin is a natural, biologically active phenylpropanoid which is extracted from the common kitchen spice nutmeg. Along with its insecticide properties, myristicin is a mild hallucinogen. Fortunately the doseage of nutmeg required to cause these effects is not the amount one normally uses in cookery! Still, the amount of myristicin in nutmeg is more than other herbs which contain the compound, such as dill and parsley.
History
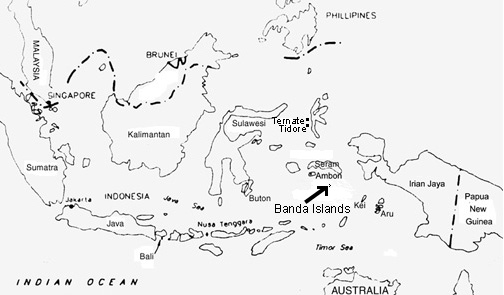
Nutmeg was first dicovered in 1512 in the Banda Islands, Indonesia by the Portuguese during an expedition to the Spice Islands. Until the 19th century, this was the only place in the world that nutmeg could be found.
From this discovery, western civilisation reaped the benefits of this strange new spice. These included cookery (of course) and also relief from sprains and rheumatism. In the 16th century it was grated and mixed with lard as an ointment for piles, but in more recent times it is used in soap, candle making, dental products and hair lotions.
In 1966 it was found that very low amounts of myristicin were found in the smoke of commercial cigarettes, but not enough to produce a significant effect on the user. [1]
Effects
Myristicin is an inhibitor of the monoamine oxidase enzyme which catalyses the oxidation of monoamines to carboxylic acids. This property is shown in all drugs given in the treatment of depression, and thus demonstrates Myristicin's medicinal properties as an anti-depressant in small dosages. [2]
Extraction
Myristicin is extracted from nutmeg oil and is one of the highest fractions when the oil is separated. The composition of nutmeg oil is 4% myristicin. The nutmeg oil itself is extracted by steam distillation of the dried seeds.
Nutmeg oil
Nutmeg oil can be bought commercially and used in aromatheraphy and massage.
Uses
Nutmeg oil stimulates the heart and circulation, activates the mind and revives people from fainting spells, while stimulating the digestive system and fighting wind, nausea, chronic vomiting and diarrhea. It encourages appetite and averts constipation, fights gallstones and is a tonic for the reproductive system, while regulating scanty periods, relieving frigidity and impotence. It can aid births by strengthening contractions. The oil has shown good anti-inflammatory action, and is also successful in relieving pain, especially muscular aches and pain, as well as rheumatism.
Precautions
Nutmeg oil is considered non-toxic, non-irritant and non-sensitizing, yet in very large dosage may become toxic with symptoms such as nausea and stupor, which is most likely due to the myristicin contained in the oil (mace oil has a higher concentration of myristicin than normal nutmeg oil). This oil should not be used during pregnancy.
Synthesis
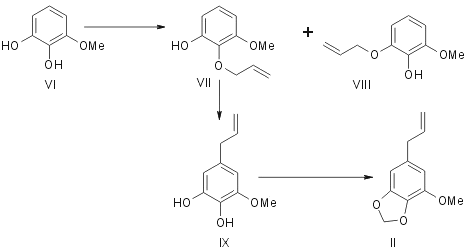
Myristicin can be synthesised from 1-methyl pyrogallol (VI). This gives a mixture of two liquid monoallyl ethers (VII and VIII). They are separated and characterised as their 3,5-dinitrobenzoates. (VII) is rearranged on pyrolysis to give 3-methoxy-4,5-dihydroxy allyl benzene (IX) which is methylenated with methylene iodide to give myristicin (II). [3]
References
- ↑ Irwin Schmeltz, R. L. Stedman et al., Science Magazine, January 1966, Vol. 151. no. 3706, pp. 96 - 97
- ↑ G. Weiss, Hallucinogenic and narcotic-like effects of powdered Myristica,Psychiatr Q., 1960, Vol. 34,346-56
- ↑ Synthesis of Myristicin and Eugenol
| Myristicin | |
|---|---|
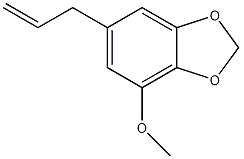
| |
| General | |
| Systematic name | systematic name |
| Other names | 6-allyl-4-methoxy-benzo[1,3]dioxole
2,3-methylenedioxy-5-(2-propenyl)-anisole 4-methoxy-6-(2-propenyl)-1,3-benzodioxole
|
| Molecular formula | C11H13O3 |
| SMILES | COC1=CC(=CC2=C1OCO2)CC=C |
| Molar mass | 192.21gmol-1 |
| CAS number | 607-91-0 |
| Properties | |
| Density & phase | 1.1425 g/cm³, liquid |
| Melting point | -300C |
| Boiling point | 1730C at 40 Torr |
| Chiral rotation [α]D | +11.31° |
| Spectral data | GC |
| Related compounds | |
| Related compounds | Myristicin aldehyde, safrole |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| <jmol>
<jmolApplet> <title>Myristicin</title><color>blue</color><size>200</size> <script>zoom 80; cpk on;frame 1; move 10 -20 10 0 0 0 0 0 3; delay 1;</script> <uploadedFileContents>Myristicin.mol</uploadedFileContents> </jmolApplet> <jmolMenu> <item><text>Start spinning</text><script>spin on</script></item> <item><text>Stop spinning</text><script>spin off</script></item> <menuHeight>-1</menuHeight> </jmolMenu> <jmolButton> <script>console</script> <text>open a console window</text> </jmolButton> | |