It07:Morphine
| It07:Morphine | |
|---|---|

| |
| General | |
| Systematic name | 7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol |
| Molecular formula | C17H19NO3 |
| SMILES | CN5CC[C@]23C1=C4C=CC(O)=C1O[C@H]2[C@@H](O)C=C[C@]([H])3C[C@@H]45 |
| Molar mass | 285.34 g/mol |
| Appearance | White Powder/Pills/Colourless liquid |
| CAS number | 57-27-2 |
| Properties | |
| Density & phase | {{{Density}}} g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | {{{Mp}}} K |
| Boiling point | {{{Bp}}} K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | {{{Hazards}}} |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Vas_morphine.pdb |
Introduction
Morphine is the main biologically active derivative of opium, which is extracted from the Papaver somniferum species of poppy. Its narcotic effect on the nervous system has led to its widespread use as an intense pain killer in hospitals and other medical care. As it is a powerful drug, it sometimes produced side-effects such as impairment of physical and mental performance, decrease in hunger, and inhibition of the cough reflex. In women it can cause disruption to the mentstrual cycle.
Addiction and Withdrawal
Being and opiate, it is very similar in structure to heroin, and thus gives rise to similar biochemistry, resulting in feelings of euphoria, and significantly reduced feelings of fear and anxiety. It is highly addictive, and both psycological and physical dependence can ensue quickly. Withdrawal symptoms include diarrhoea, stomach cramps and insomnia amongst others.
Synthesis
Morphine can be synthesised either biologically or via numerous artificial methods. The biological pathway begins with the amino acid L-tyrosine, which is converted through 2 steps into (S)-norcoclaurine. This is then alkylated to give a product called (S)-reticuline, which is oxidised to form a 1,2 dehydroreticulinium salt the salt is then reduced, and the stereocentre inverted, by hydrogenation using the naturally occuring molecule NADPH, which is then oxidised to NADP. The desired product from this reaction (R)-reticuline, is unstable due to phenolic coupling, and quickly forms salutaridine. A reduction of the ketone group then ensues, converting the product into salutaridinol which can then undergo SN2 in acidic conditions. The mechanism for this process is shown below:
The methoxy group is then converted from an ether into a ketone group to form neopinone. A proton transfer then occurs to form codeinone before the newly formed ketone group is reduced to a hydroxyl group. This final product is Morphine. The full synthesis is displayed below.
The landmark artificial synthetic method was developed by M.J. Gates[2] and since then other syntheses such as the Rice[3] synthesis and Overman[4] synthesis amongst others have been developed and used to synthesise morphine from a variety of starting materials.
History
Used as a pain relief medicine during the American Civil War, morphine was widely employed, resulting in many soldiers having the 'war disease' morphine addiction when the war ended. In its early life it was prescribed by some physicians to cure the problem of alcohol addiction, as morphine was considered to be less harmful to the body, and produced less anti-social behaviour. Despite being made illegal in the UK by the Harrison Narcotics Act in 1914, morphine remained a popular recreational drug until heroin was developed (originally as a cure for morphine addiction)[5].
Spectroscopic Data
Infra-red Spectrum
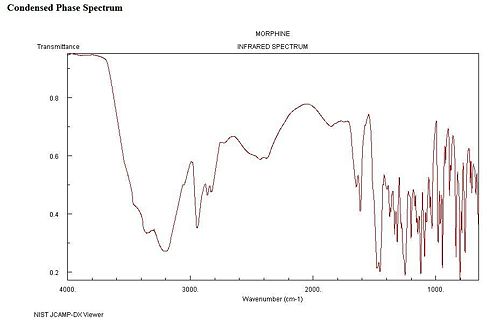
Mass Spectrum

References
- ↑ Taber, D.F. The Enantioselective Synthesis of Morphine: Strategies and Tactics in Organic Synthesis 2004, 5, 353
- ↑ Gates, M.J. J. Am. Chem. Soc. 1953, 75, 4340
- ↑ Rice, C.; Brossi, A. J. Org. Chem. 1980, 45, 592
- ↑ Overman, L.E. Pure and Appl. Chem. 1994, 66, 1423
- ↑ The Columbia Electronic Encyclopedia, 6th ed. Copyright © 2007, Columbia University Press. All rights reserved.
- ↑ NIST (National Institute of Standards and Technology)
- ↑ NIST (National Institute of Standards and Technology)


