It07:Methoxsalen
Methoxsalen
| Methoxsalen | |
|---|---|
| |
| General | |
| Systematic name | 9-methoxy-furo[3,2-g]chromen-7-one |
| Other names | Ammodin
Ammoidin Meladinin (VAN) Meladinine Meladoxen Meloxine Methoxa-Dome Methoxalen Methoxsalen New-Meladinin NCI-C55903 Oxoralen Oxsoralen Oxypsoralen Puvalen Xanthotoxin Xanthotoxine XANTHOTOXIN 5-Benzofuranacrylic acid, 6-hydroxy-7-methoxy-, -lactone {7H-Furo[3,2-g][1]benzopyran-7-one,} 9-methoxy- ( ) 8-Methoxy(furano-3'.2':6.7-coumarin) {8-Methoxy-[furano-3'.2':6.7-coumarin]} 8-Methoxy-2',3',6, 7-furocoumarin 8-Methoxy-4',5',6,7-furocoumarin 8-Methoxypsoralen 8-Methoxypsoralene 8-MOP 8-MP |
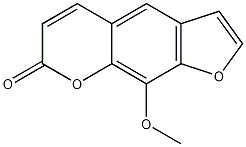
| Molecular formula | C12H8O4 |
| SMILES | COC2=C1OC(C=CC1=CC3=C2OC=C3)=O |
| Molar mass | 216.1928gmol-1 |
| Composition | C 66.67% H 3.73% O 29.60% |
| CAS number | 298-81-7 |
| Properties | |
| Density & phase | {{{Density}}} g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | 416.15K |
| Boiling point | {{{Bp}}} K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | {{{Hazards}}} |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Hydrogen Bond Donor Count |
0 |
| Hydrogen Bond Acceptor Count |
4 |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Methoxsalen is a drug that naturally occurs in Ammi majus (Umbelliferae) seeds and in the roots of Heracleum Candicans, and is part of a group of coumpounds known as psoralens. It is used to treat vitiligo and psoriasis, along with other medical conditions and procedures, such as the treatment of white blood cells during photopheresis. Vitiligo, a disease in which skin pigment is lost, and psoriasis, a skin condition which causes red and scaly patches on the skin, are both treated by a procedure called PUVA, which involves the patient taking the methoxsalen drug prior to ultraviolet light exposure(UV-A). The drug is taken 2 hours before the patient undergoes the UV therapy. Once inside the body, methoxsalen is excited by the UV radiation, and acts on the skin cells by selectively inhibiting DNA synthesis. It does this by binding covalently to the pyrimidine bases, guanine and cytosine, causing cross linking of DNA, inhibiting its synthesis and function.

|
|---|
| http://www.electroherbalism.com/images/regimens/Vitiligo.JPG |
Side Effects
Nausea is a very common side effect of taking this drug, as it occurs with approximately 10% of all patients. This is usually minimised by taking the drug with milk or food, or splitting the dose in half and having it in two portions 30 minutes apart. Other side effects include damage to the eye, cataracts, skin cancer and skin ageing, due to the use of UV light along with the treatment. Methoxsalen causes changes to skin colour.
Other Uses
The most famous use of Methoxsalen was by an author, John Howard Griffin (Dallas, Texas), who in 1959 used the drug to make himself appear black, so that he could find out first hand what it was like to experience discrimination based on skin colour. Griffin went to a dermatologist, who agreed to give him an accelerated dose of the drug with UV treatment, and this, along with small amounts of dye, darkened his skin colour dramatically. Griffin then started a 6 week journey through South America, including Louisiana, Mississippi, Georgia and Alabama, and initially recorded his experiences in an article which was published in a prominent black magazine, Sepia. Later, he wrote of his journey in greater detail in his book “Black Like Me”. Following Griffin’s death in 1980, rumours followed, suggesting his cause of death being due to his skin darkening for his experiment. However, it was in fact due to diabetes related complications.
3D rendering of Methoxsalen
Synthesis
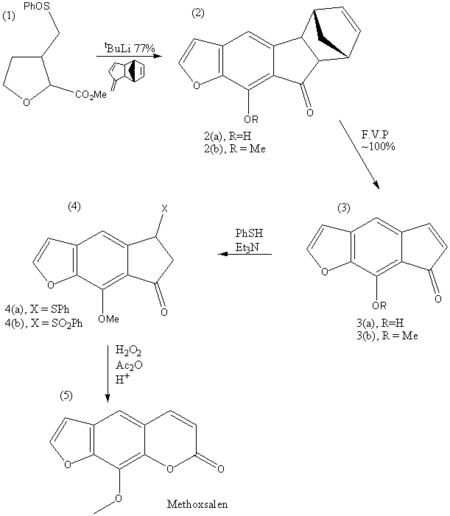
The synthesis of methoxsalen starts with (1), flurosulfoxide, which undergoes annulation with the molecule shown in the presence of tBuOLi. This gives two products as shown (2a and 2b). The next step involves Flash Vacuum Pyrolysis (F.V.P. - 500*C, 0.1mm)in which oxaindacenones are produced from 2(a) and 2(b) to 3(a) and 3(b) respectively. The next step involves treatment of 3(b) with thiophenol in the presence of triethylamine, which gives the product 4(a). This is followed by the oxidation of 4(a) which yields methoxsalen (20%) and 4(b). 4(b) can then be converted to 3(b) by DBU treatment,which is then further oxidised to give a higher yield of methoxsalen.
Spectral Data
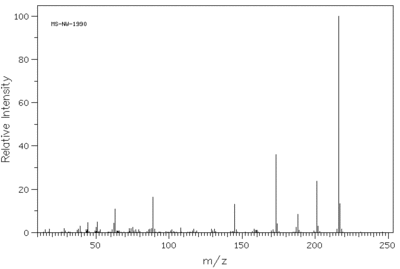
Mass Specrometry for the molecule Methoxsalen(molecular formula C12H8O4)
http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ms&sdbsno=10625
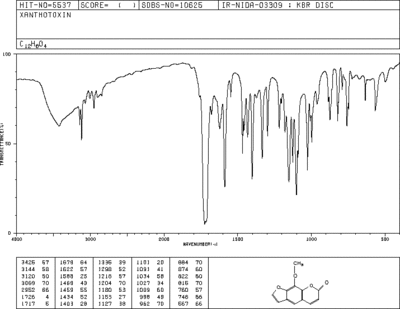
Infra Red Spectrum of Methoxsalen
http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ir&sdbsno=10625
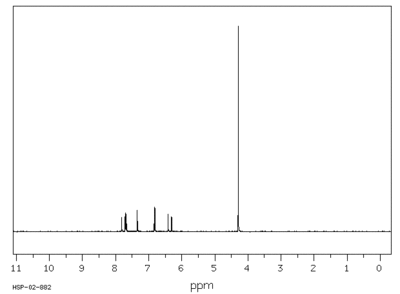
H1NMR of methoxsalen
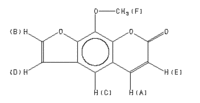
| Assignment | Shift (ppm) | |
|---|---|---|
| A | 7.764 | |
| B | 7.689 | |
| C | 7.346 | |
| D | 6.818 | |
| E | 6.363 | |
| F | 4.293 |
J(A,E)=9.6HZ. J(B,D)=2.2HZ.
http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=hnmr&sdbsno=10625
References
1. D. Mal, K. V. S. N. Murty and K. Datta, Tetrahedron Letters, 1994, 35, 9617-9618.
http://chemfinder.cambridgesoft.com/result.asp http://www.medicinenet.com/methoxsalen-oral/article.htm http://www.mongabay.com/health/medications/Oxsoralen-Ultra.html http://129.43.27.140/ncidb2/ http://www.chemcalc.org/ http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi http://www.snopes.com/horrors/freakish/griffin.asp http://www.washingtonpost.com/wp-dyn/content/article/2007/03/16/AR2007031602173.html http://www.rxlist.com/cgi/generic/methoxsalen.htm