It07:Menthol
Menthol
| Menthol | |
|---|---|

| |
| General | |
| Systematic name | systematic name |
| Other names | (1S)-menthol
(1S)-(1rH,4tH)-p-menthan-3t-ol (+)(1S)-Menthol (+)-menthol (1S,2R,5S)-(+)-menthol |
| Molecular formula | C10H20O |
| SMILES | O[C@@H]1C[C@H](C)CC[C@H]1[C@@H](C)C |
| Molar mass | 156.27gmol-1 |
| Composition | c%H%O% |
| CAS number | 89-78-1, 490-99-3, 491-01-0, 491-02-1, 1490-04-6(racemic mixture), 2216-51-5, 2216-52-6, 3623-51-6, 3623-52-7, 3623-53-8, 15356-60-2, 15356-70-4, 20747-49-3, 20752-33-4, 20752-34-5, 23283-97-8, 64282-88-8 |
| Properties | |
| Density & phase | 0.891 g/cm³, solid |
| Melting point | 41-420C |
| Boiling point | 103-1040C |
| Chiral rotation [α]D | 48° |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Irritant,Flammable |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Hydrogen Bond Donor Count |
|
| Hydrogen Bond Acceptor Count |
|
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Menthol |
Menthol is the chemical responsible for giving peppermints (and mint it's self) their cool sensation. It has the chemical formula C10H20O and has a crystalline structure.
Synthesis
The synthesis of synthesis of a racemic mixture of (S)-menthol (shown above) and (R)-menthol was devised by Hueckel and Bretschneider and published in 19381. Ethyl sodium, cyclohexane and ethyl-((1R)menthyl)-ether were used as the starting materials, yielding racemic menthol with ethane and 2-ethyl-1-isopropyl-4-methyl-cyclohexane as side products. A newly devised mechanism (winning the Nobel Prize in 2001!), to obtain optically pure (-)-menthol is shown below, using a chiral catalyst.
2
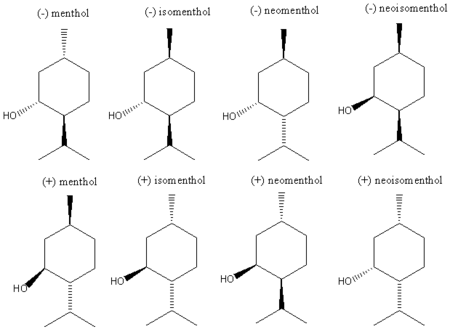
Stereoisomers
In addition to natural (-)menthol which is primarily produced by biosynthesis in nature, there are 7 other stereoisomers of the molecule, as shown below.
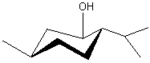
Chair conformation of (-)menthol:
Spectra
Infra Red
Mass Spectrum
 Menthol is now synonymous with cigarettes
[3]
Menthol is now synonymous with cigarettes
[3]
References
1Hueckel and Bretschneider, J. Prakt. Chem., 1938, <2> 151, 61, 62. 2http://nobelprize.org/nobel_prizes/chemistry/laureates/2001/noyori-lecture.pdf