It07:Melem (Melon)
| It07:Melem (Melon) | |
|---|---|

| |
| General | |
| Systematic name | 1,3,4,6,7,9,9b-Heptaazaphenalene |
| Other names | tri-s-triazine, cyamelurine |
| Molecular formula | C6N7(NH2)3 |
| Molar mass | 218 |
| Appearance | fine, pale yellow powder |
| CAS number | 1502-47-2 |
| Properties | |
| Density & phase | 1.717 g/cm³ |
Melem, or Melon (the polymeric form) is the novel name for Tri-s-triazines, or a functional group of C6N7 triangular. The melem group of compounds are relatively unreactive due to large amount of resonance that can occur throughout the system.
History
The Melems were first discovered by Jöns Jakob Berzelius in the 1830s,when he found the substance after igniting mercury thiocyanate. The polymer melon was later named by Justus VonLiebig.
Specific Melems and Their Uses
The Melem compound itself is used as a fire retardant[1]. This is done by preventing either preventing ignition, this is done by causing a heat sink through thermal decomposition (at a high temperature). The nitrogen produced through this decomposition is inert, and so can act as a inert diluting agent. The Melem can also create a barrier between oxygen and other gases, being stable means that this barrier will not break down. Melem triangles can be joined together by of a C-N-C bond or C-N-N bond to give a range of polymers[2].
Structure
Melem |
Synthesis
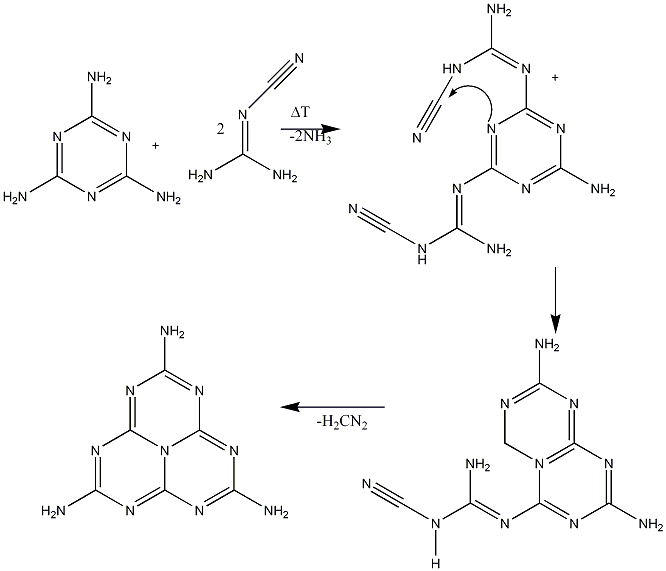
2 possible synthesises have been suggested for Melem[3], both involving the condensation of melamine ringsMelem can be synthesised through two main methods; through the condensation of melamine rings:
Possible Mechanism 1:

Possible Mechanism 2:
References
- ↑ Flame retardant properties of melem
- ↑ The First Synthesis and Characterization of Cyameluric High Polymers, Tamikuni Komatsu
- ↑ Melem (2,5,8-Triamino-tri-s-triazine), an Important Intermediate during Condensation of Melamine Rings to Graphitic Carbon nitride: Synthesis, Structure Determination by X-ray Powder Diffractometry, Solid state NMR and Theaoretical Studies, Barbara Jurgens et al, 2003
