It07:Mefloquine
Mefloquine (Lariam)
| Mefloquine | |
|---|---|
[[Image: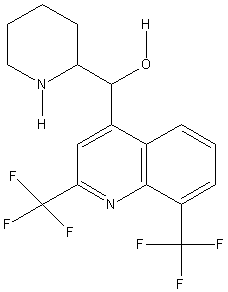 |250px|Mefloquine]] |250px|Mefloquine]]
| |
| IUPAC Systematic name | |
| [2,8-bis(trifluoromethyl)quinolin-4-yl]-(2-piperidyl)methanol | |
| Other name | |
| mephaquine&btnG=Google+Search&meta= lariam, mephaquine | |
| Indentifiers | |
| ATC Code | P01BC02 |
| CAS number | {{{CASNo}}} |
| PubChem (CID) | 4046 |
| SMILES | C1CCNC(C1)C(C2=CC(=NC3=C2C=CC=C3C(F)(F)F)C(F)(F)F)O |
| Chemical Data | |
| Molecular formula | C17H16F6N2O |
| Molar mass | 378.312 g/mol g/mol |
| Pharmacokinetic Data | |
| Bioavailability | <85% |
| Protein Binding | 98% |
| Metabolism | mostly hepatic |
| Half life | 305.3hrs[1] |
| Excretion | Mostly via the faeces and bile. Urinal excretion composed of 9% unchanged mefloquine; 4% main metabolite |
| Therapeutic considerations | |
| Pregnancy cat. | C (US) |
| Legal status | Approved drug |
| Routes | Oral |
Mefloquine, better known as Lariam (produced by Hoffman La-Roche pharmaceuticals) is an orally-administered drug that is used for treatment, as well as a prophylactic measure against malaria. It was developed in the 1970's in the Walter Reed Army Institute of Research, USA, as a synthetic analog of the naturally-occuring molecule,quinine (found naturally in the Peruvian cinchona tree).
3-D image of the molecule
Hyoscine |
A short overview on Malaria
Malaria is transmitted from person to person, by female anopheles genus mosquitoes that are infected with the plasmodium parasite. This parasite then multiplies and matures in the liver before reentering the blood stream and attacking red blood cells. Malaria is widespread in tropical and subtropical regions, where the climate is favourable for the breeding of this mosquito.[2]
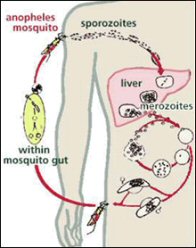 Taken from www.traveldoctor.co.uk/malaria.htm
Taken from www.traveldoctor.co.uk/malaria.htm
Symptoms include: fever, shivering, vomiting, joint poin, amaemia, convulsions, haemolysis (caused by the lysing of the red blood cells)and haemoglobinuria (this is when excess haemoglobin is excreted via the kidneys).
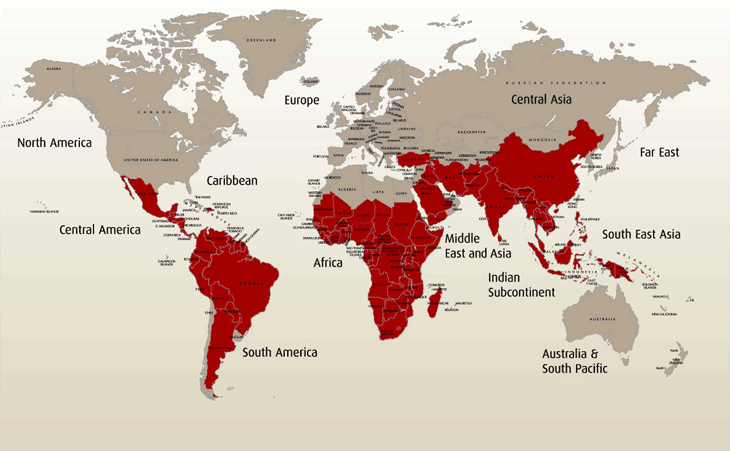
This is a map of where malaria is commonly found:
Taken from http://www.malariahotspots.co.uk/files/images/MH_L1_facts_map.gif
Physical Properties
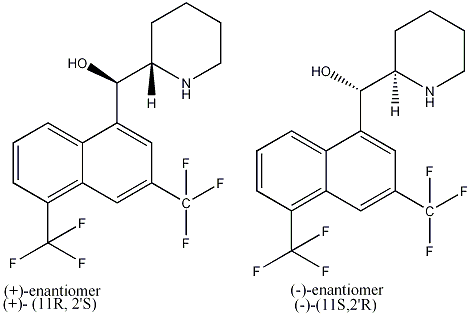
Mefloquine is a white- white crystalline solid that is slightly soluble in water. It is derived from 4-quinolinemethol and has the specific chemical name of (R*,S*)-(+_)-α-2-piperidinnyl-2,8-bis(trifluoromethyl)-4quinolinemethanol hydrochloride. Mefloquine hydroxide is a chiral molecule and the drug contains both of these enantiomers. It is still not known whether there is stearic switching after the drug has been consumed. It has been found, however that not only does the (-) enantiomer have a shorter halflife, but it also binds to the adenosine receptors in the central nervous system [3], which may explain some adverse side-effects of the drug.[4]
More about the Drug
Mefloquine is a schizonticide- a drug that selectively targets a certain sporazoan parasite. The exact mechanism of this remains unknown. It is possible that the drug forms toxic haem complexes with free haem in the blood that then go on to damage the membrane and other compenents of the parasite. It is classified within the quinine family that also includes Chloroquine, quinine, quinidine and Hydroxychlorquine. Mefloquine is effective when treating chloroquine-resistant strains of malaria and is active during the time the parasite is in the blood (erythrocytic period). However, it has no effect during the hepatic stages of the illness.[4]
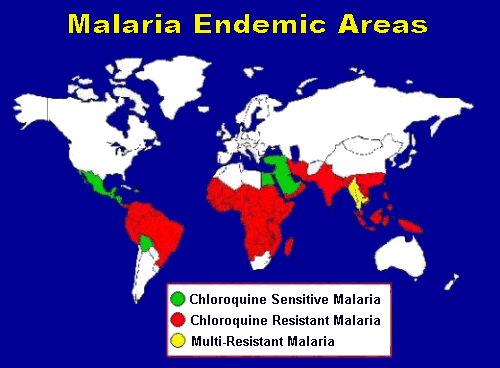
Mefloquine Resistance
The parasite responsible for malaria is growing ever-more resistant to antimalarial drugs, which makes treatment and prevention more difficult. Strains of the parasite with increased resistance to mefloquine have been found in South East Asia, notably in Myanmar, Thailand and Cambodia, as well as incresingly in other parts of the world. Cross resistance of mefloquine and quinine, as well as mefloquine and halofantrine (a non-prophylactic antimalarial drug that is only used in treatment) has also been observed in other regions. Treating drug-resistant malaria requires a mixture of different treatments.[2]
From www.traveldoctor.co.uk/malaria.htm
How to use lariam
Prophylactic treatment starts one week before travelling to a malaria zone. After that, one pill of 250mg (provided you are over 45kg in weight) is taken once a week. The treatment should only be ended 4 weeks after returning from the endemic area. The pills should be washed down with plenty of water and never taken on an empty stomach.[1]
Side Effects
The side effects for using mefloquine as a prophylactic measure, as well as a treatment are similar. They include: vomiting, nausea, tinnitus, dizziness, headaches, fatigue, loss of appetite, abdominal pain, diarrhoea, chills, loose stools, vertigo, insomnia and vivid dreams. More severe (and rare) side effects include: aggression, depression, mood swings, hallucinations, encephalopathy (disease of the brain resulting in a change of brain structure), psychosis, confusion and paranoia. There have been reports that the use of mefloquine slightly increases the incidence of suicide.[1]
It is generally unadvisable for those to take lariam, who have a previous history of any kind of a mental condition.
Mefloquine and Pregnancy
Women are advised to avoid becoming pregnant, while taking mefloquine, as the drug is suspected to increase the probability of a stillbirth occuring, particularly during the first trimester.[5]
References
[1] Complete Product Information- Roche Pharmaceuticals
[2] [www.traveldoctor.co.uk/malaria.htm Travel Doctor]
[3] (-) Mefloquine to block puringergic receptors
[4]Drug Bank