It07:Mefenamic Acid
| It07:Mefenamic Acid | |
|---|---|
| |
| General | |
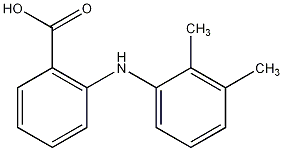
| Systematic name | 2-(2,3-dimethylphenyl)aminobenzoic acid |
| Molecular formula | C15H15NO2 |
| SMILES | CC1=C(C(=CC=C1)NC2=CC=CC=C2C(=O)O)C |
| CAS number | 61-68-7 |
| PubChem Identifier | Substance: 148984 - Compound: 4044 |
| Properties | |
| Molar mass | 241.29 gmol-1 |
| Melting point | 230-231oC |
| Boiling Point | 398.8 +/- 30.0oC |
| Density | 1.203 +/- 0.06 gcm-3 |
| Solubility in water | 20mgL-1 |
| Vapour Pressure | 4.45x10-7Torr |
| Flash Point | 195.0 +/-24.6oC |
| Enthalpy of Vaporisation | 68.49 +/- 3.0 kJmol-1 |
| Logp/Hydrophobicity | 4.041 |
| pKa/Isoelectric Point | 4.2 |
| Elimination Half Life | Two Hours |
| Protein Binding | 90% |
| Hazards | |
| Material Safety Data Sheet | Click Here for MSDS File (html) |
| Side Affects of Oral Use - Overdose | Skin Reactions - Stomach Pain-Nausea - Irregular Heartbeat - Ringing in Ears - Dark Stool - Muscle Weakness - Confusion - Fever |
| Overdose | 740 mgkg-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Introduction
Mefenamic acid is an anthranilic acid derivative[1] and is a member of the fenamate group of non-steroidal anti-inflammatory drugs (NSAIDs).
Mefenamic acid has important uses as a pharmaceutical drug acting as an anti-inflammatory, which has particular effectiveness in treatment of ‘heavy’ menstruation[2] , under the commercially named Ponstel® and many other names. Other major uses include treatment of rheumatoid arthritis, osteoarthritis and dysmenorrheal. It can also be prescribed for antipyretic purposes[3] during times of elevated body temperature, for example, treating bad cases of fever.
The bodily treatment pathway is for the hypothalamus[1] (region of the brain that links the nervous system to the pituitary gland and the endocrine system) to block a white blood cell induced temperature rise due to foreign stimuli. The ability of Mefenamic acid to treat inflammation and uterine contractions is thought to be due to inhibition of hormones such as prostaglandin[4], however a mechanism for this process is still under debate. One possible theory is that the Mefenamic acid binds to the enzyme that synthesises prostaglandin (prostaglandin synthetase) at the receptor sites named COX-1 and COX-2, which function as major mediators to the inflammation process.
Removal, or chemical elimination, of this molecule is important due to its side effects (see below). As hepatic metabolism is important in this removal process patients with liver problems may require smaller doses to avoid accumulation. Renal defects could also potentially cause build-up of Mefenamic acid and its metabolised products which is the reason why patients with kidney irregularities should not be prescribed Mefenamic acid[1]. The metabolic products of Mefenamic acid are 3-hydroxymethylmefenamic acid and further such metabolism (oxidation) produces 3-carboxymefenamic acid, the images of which are shown below.
Dosage[5]
Doses of 500mg tablets, orally administered, are typical for acute pain and treatment of rheumatoid and osteo-arthritis, which is then boosted by a further 250mg every 6 hours with usually a daily limit of 1000mg for milder cases.
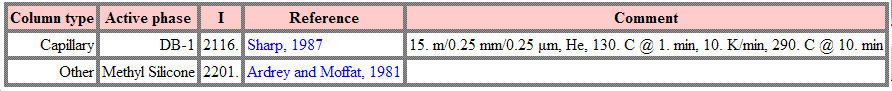
Analysis of the dosage and drug in general can be made possible by use of HPLC techniques (high performance liquid chromatography) which is an effective type of column chromatography that can separate a large number of components via a range of molecular interactions that may or may not be exhibited by the target molecule. Modern techniques utilise infra-red detection with a reverse phase column for excellent reproducibility and sensitivity.
Side Effects and Toxicology[1]
Cases of drowsiness, headache and digestive problems have been linked directly as effects of using this drug. For obvious reasons therefore, it is highly advisable to avoid such activities as driving or any type of drug inebriation.
Less common side effects of use of this drug include nervousness, nausea which can include bloody vomiting, vision impairment, skin complaints and ironically fever. Clearly these effects all have a range of seriousness and if any of these symptoms are encountered whilst taking the drug please seek medical clarification. Below is a toxicology report[6] for chemical reference purposes and medical interest.
Reactions
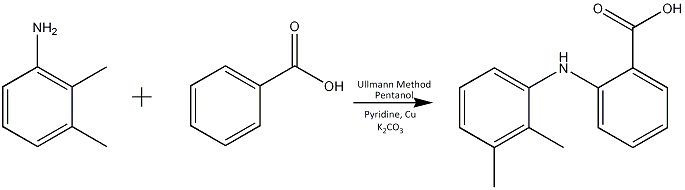
There are many synethetic routes of making Mefenamic acid, one of which involves the so-called modified Ullman method after the German chemist Fritz Ullman (1875-1939) who also introduced dimethyl sulphate as an alkylating agent. The Ullman method is notorious for requiring harsh and specific conditions as can be seen from the reaction scheme below. This method can be classified as a coupling reaction between aryl halides requiring copper and a possible mechanism is via the formation of an organocopper halide intermediate which reacts with the aryl halide in a nucleophilic aromatic substitution reaction. The modification is to not use sand as the medium for the reaction and this type of reaction can have very erratic yields.
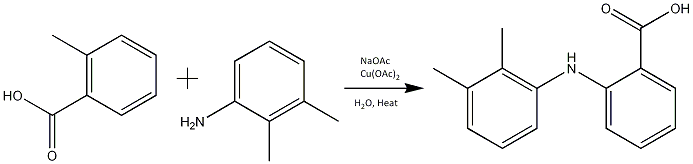
An alternative synthesis to the one above (of which there are many) can involve copper as a catalyst, present as Cu(OAc)2 (Cupric Acetate) this reaction has a reliably high yield of 93%[7].
Biological reactions such as reductive and oxidative processes of Mefenamic acid are important as they allow the molecule to have a pharmaceutical use as mention above and removed via excretion in the urine and fecal matter. Conjugation occurs to Glucuronic acid and the excreted products consist of a mixture of conjugates of metabolised Mefenamic acid. Typical excretory percentages of dosage above is; 50% conjugated Mefenamic acid, 25% is conjugated 3-hydroxymefenamic acid and 20% is a mixture of conjugated and free 3-carboxymefenamic acid. Reaction schemes for metabolic oxidation of Mefenamic acid are shown below.

Analytical Data
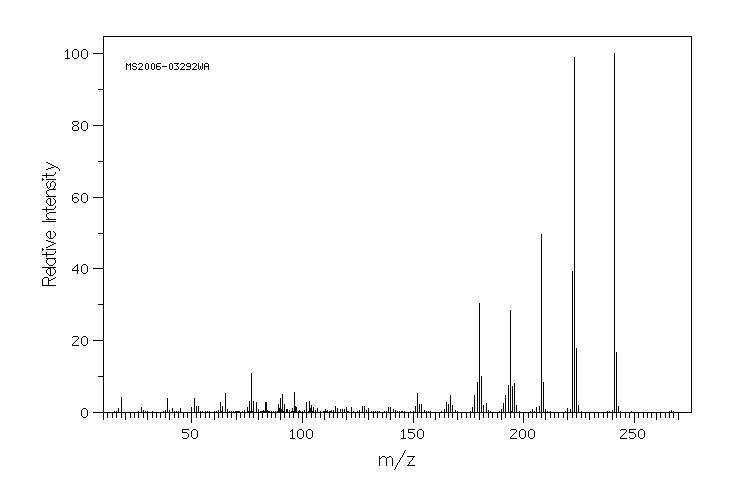
The mass spectrum[8] and some referenced gas chromatography data[9] are shown below for Mefenamic acid. The mass spectrum shows the molecular ion peak at approximately the relative molecular mass of the compound and approximately half the relative molecular mass of Mefenamic acid. The fragments that are cearly identifiable include Me+ groups (methyl) of fifteen units (m/z) and +COOH (formic acid).
References
- 1: http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=APRD00730.txt
- 2: http://www.medicinenet.com/mefenamic_acid-oral/article.htm
- 3: http://www.medind.nic.in/imvw/imvw355.html
- 4: http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=EXPT01314.txt
- 5: Analytical Toxicology for Clinical, Forensic and Pharmaceutical Chemists – Hans Bradenberger, Robert A. A. Maes (link to google books)
- 6: http://redpoll.pharmacy.ualberta.ca/drugbank/drugBank/drugFile/msds/APRD00730.html
- 7: Journal; Girisha, Hanakere R.; Srinivasa, Gejjalagere R.; Gowda, D. Channe; JRPSDC; J. Chem. Res. Synop.; EN; 5; 2006; 342 - 344.
- 8: http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi
- 9: http://webbook.nist.gov/cgi/cbook.cgi?Name=mefenamic+acid&Units=SI
