It07:Lysergic Acid
| It07:Lysergic Acid | |
|---|---|
| |
| Chemical name | 6-Methyl-9,10-didehydro- ergoline-8-carboxylic acid or 7-methyl-4,6,6a,7,8,9- hexahydro-indolo[4,3-fg] quinoline-9-carboxylic acid |
| Chemical formula | C16H16N2O2 |
| Molecular mass | 268.31 g/mol |
| Melting point | 238 - 240 °C |
| Properties | Prisms from methanol, dec 242°. |
| CAS number | 82-58-6, 478-95-5, 6915-32-8, 23953-76-6, 68985-97-7, 68985-98-8 |
| SMILES | O=[C@](O)[C@H]1CN(C) [C@](C2=C1)([H])CC3=C NC4=C3C2=CC=C4 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Overview
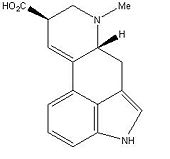
Lysergic acid, also known as D-lysergic acid, belongs to the ergometrine group of Ergot Alkaloid, It is naturally produced by a ergot fungus Claviceps Purpurea. This fungus grows on rye. It is used in pharmaceutical to make LSD by makind the lysergic acid diethylamide which is LSD., it can also be used to make ergotamine, which is used to cause contractions of the uterus following childbirth. Lysergic acid is a chiral with two stereocenters.
3D Structure
Lysergic Acid |
Salem Witchcraft Trials-1692

The Salem witch trials were a series of trials to prosecute people accused of witchcraft in Essex, Suffolk and Middlesex Counties of colonial Massachusetts, in 1692 and 1693.
Nowadays the common theory, which was first develop by Linda R. Caporael, was people had eaten bread that was made from infected rye grain that containing ergot alkaloid, the alkaloid caused the hallucinations, convulsion, burning sensation. Which is consistent with most of the physical symptoms of those afflicted by witchcraft
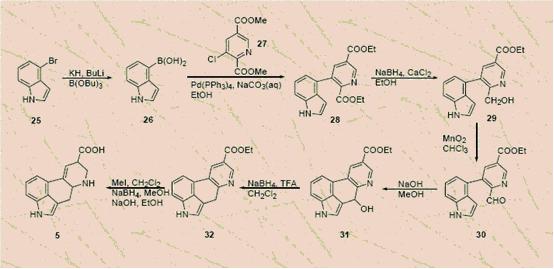
Synthesis
Woodward synthesis The first synthesis was made by Woodward and his group in 1956, it Consists of 15 steps and the starting material is 3-carboxyethylindole.
Image taken from http://www.tkk.fi/Yksikot/Orgaaninen/pihko_group/Korsoff_Feb2004.pdf
Hendrickson-Wang synthesis A shorter synthesis was developed in 1993 with 4-bromoindole and isocichomeronic acid as the starting materials
Image taken from http://www.tkk.fi/Yksikot/Orgaaninen/pihko_group/Korsoff_Feb2004.pdf
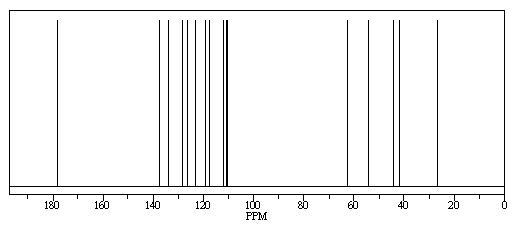
predicted NMR using chemdraw
Referemce
http://www.world-of-fungi.org/Mostly_Medical/Ziad_Madlom/Ergot_alkaloids.htm http://www.tkk.fi/Yksikot/Orgaaninen/pihko_group/Korsoff_Feb2004.pdf