It07:Limonene
Limonene
Introduction
| It07:Limonene | |
|---|---|
 [1] [1]
| |
| General | |
| Systematic name | p-mentha-1,8-diene or 4-isopropenyl-1-methylcyclohexene |
| Other names | Methyl-4-isopropenyl cyclohexene, Cajaputene, Carvene, Cinene, Dipentene, Efchole,
isopropenyl-1-methyl-1-cyclohexene, Dextro-limonene, (R)-Limonene, (R)-(+)-Limonene, 1-methyl-4-(1-methylethenyl)-, Unitene, (L)-Limonene, Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (4S)-, alpha-Limonene, beta-Limonene |
| Molecular formula | C10H16 |
| SMILES | CC(=C)[C@@H]1CCC(=CC1)C |
| Molar mass | 136.2 g/mol |
| Appearance | Light yellow liquid |
| CAS number | 5989-27-5 |
| Properties | |
| Density & phase | 0.841 g/cm³ |
| Melting point | -96.9OC |
| Boiling point | 176OC |
| Chiral rotation [α]D |
[α]20/D +115.5±1°, c = 10% in ethanol |
| Hazards | |
| MSDS | http://physchem.ox.ac.uk/MSDS/LI/limonene.html |
| Main hazards | Respiratory, skin and eye irritant. Harmful if swallowed. |
| Flash point | 50°C |
| R/S statement | R: R10 R22 R36 R37 R38 S: S28 S37N S60 S61? |
Limonene is found in abundance in the essential oils of citrus fruits (hence the name limonene, which derived from lemon rind), particularly in orange and lemon peel. Specifically, limonene is found in abundance in the oil sacs of the rind, which may be located in the flavedo, coloured or outer portion of the peel. D-Limonene takes up 90-95% of the oil, in citrus fruits. It is also found in the oils of pine trees.
Uses of Limonene
Nowadays, limonene is used in order to create an orange fragrance for various products, and it is also used in the formation of paint solids. D-limonene is divided into two components, [3] food grade and technical grade. WAhen the juice is extracted from the peel of the citrus fruit, the essential oils (mentioned above) are obtained from the juice by distillation. The final product is food grade D-limonene. This type of D-limonene is used primarily in certain consumer products. Technical grade D-limonene is obtained in a slightly different way, although a type of distillation is indeed used. A steam extractor is used to obtain the oil from the citrus fruit rind, and on condensation of the steam a layer of oil is left, floating on the water's surface. This is 90-98% technical grade D-limonene, and the rest is a combination of other monoterpenes, such as 3-carene. Technical grade D-limonene has many applications in industrial products. In the 1980s, citrus limonene began to be used instead of common household cleaning solvents. D-limonene in itself is a solvent, and it can replace various other solvents such as toluene or mineral spirits. In the house, it may be added to a surfactant and diluted to replace many caustic cleaners, for example wipe=cleaners or sprays containe D-limonene. Because it is biodegradable, it is even used as a solvent for silicones.
Limonene may be used as a component in
- graffiti remover
- hand cleaner
- car wash solution
- industrial metal cleaner
- recycling
- insect repellent
- dog and cat repellent
There are other derivatives of limonene that are used in every day products, for example carvone is used in spearmint oil (found in dental care sweets and other products).
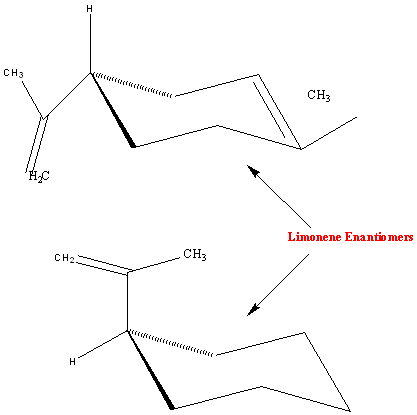
L-limonene - the [S]-enantiomer, with a turpentine-like odour.
 [4]
[4]
Structure of Limonene
The molecule is a hydrocarbon, with the molecular formula C10H16, and it is specifically a terpene. Many everyday household products, for example fruit juices or spices, contain essential oils which are part of the terpene family. The common structural characteristic of all terpenes is that they generally contain at least five, or multiples of five carbon atoms, joined together in this fashion:
The molecule is chiral, which means that it exists as two enantiomers with different characteristics. D-limonene, the (R)-enantiomer, has an orange scent and L-limonene, the (S)-enantiomer, has a smell reminscent of turpentine.
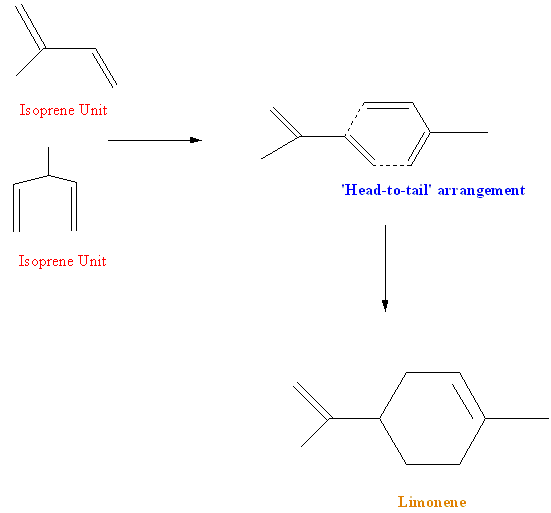
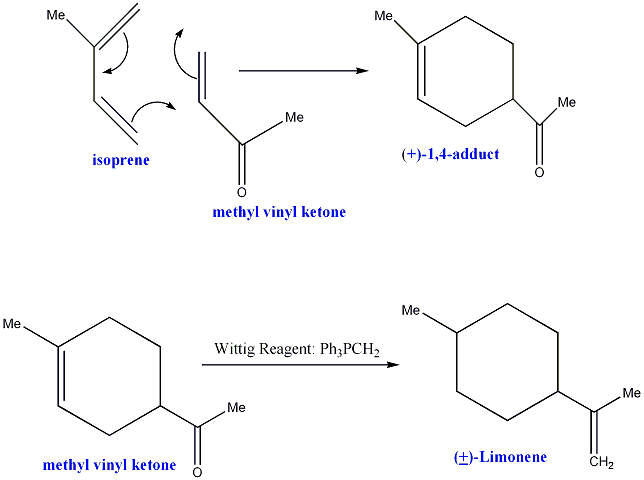
Mechanism of Formation
Medicinal Properties
D-Limonene is used as a supplement to treat and prevent cancer. As described above, D-limonene is a ‘‘terpene’’, specifically a ‘‘‘cyclic monoterpene’’’. These structures lead to an alteration of a gene expression, from the prevention of the isoprenylation of G proteins. The result of this is a G1 cell cycle arrest, apoptosis (a type of PCD, or Programmed Cell Death), and eventually the replacement of tumor parenchyma[5] by stromal (organ framework) elements, which are predominantly made of connective tissue. Monoterpenes prevent the carcinogenesis process during all the stages, and are thought to be efficient in treating cancers in all periods. D-Limonene metabolises, after oral administration, to form limonene-1,2-diol, dihydroperillic acid, perillic acid and uroterpenol, all of which have the same effects outlined as above. Limonene also promotes the Gluthione-S-transferase (GST) system [6] in the small bowel and liver, which detoxifies carcinogens (cancer-promoting substance, for example benzopyrene, found in cigarette smoke).
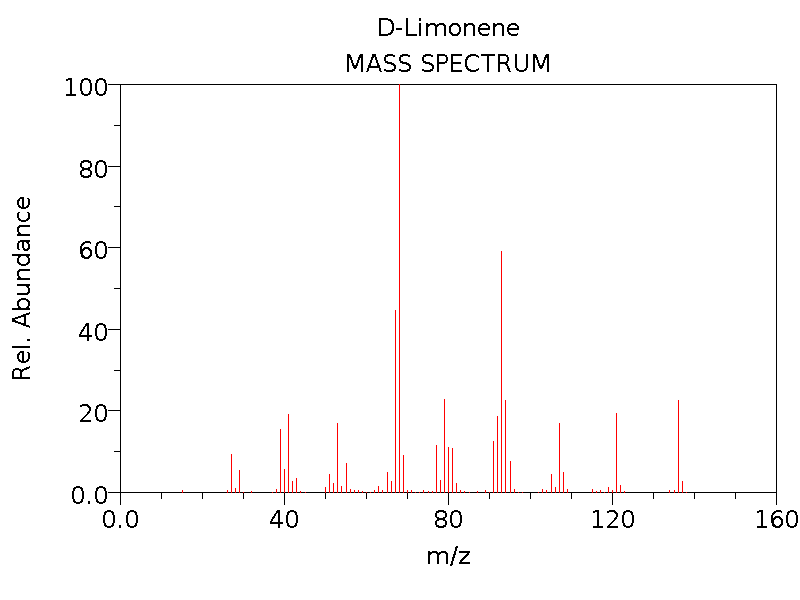
Spectroscopy
Molecule-1 |
References
- ↑ [www.3dchem.com/imagesofmolecules/Limonene.jpg] www.3Dchem.com
- ↑ SciFinder
- ↑ [1] Biochem Corp. of Florida, Inc.
- ↑ [2] Lyondell - A Leading Global Producer of Chemicals, Fuels and Plastics.
- ↑ [3] ehp Environmental Health Perspectives
- ↑ [4] Memorial Sloan-Kettering Cancer Centre
- ↑ [5] NIST Chemistry WebBook
- ↑ [6] NIST Chemistry WebBook
- ↑ SciFinder