It07:Lignocaine
Lignocaine
| It07:Lignocaine | |
|---|---|
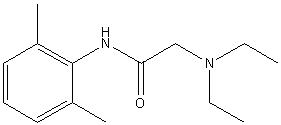
| |
| General | |
| Systematic name | 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide |
| Other names | Lignocaine, L-Caine, Lidocaine(VAN), Maricaine, Ruicana, Solcain, Xylocaine, Xylocard, Xylestesin, Xylocitin |
| Molecular formula | C14H22N2O |
| SMILES | CC1=C(NC(CN(CC)CC)=O)C(C)=CC=C1 |
| Molar mass | 234.17 gmol-1 |
| CAS number | 137-58-6 |
| Properties | |
| Melting point | 339-342 K |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Lignocaine is the British Approved Name of the drug known as Lidocaine under its International Nonproprietary Name. It was the first type of amino amide-type local anaesthetic in medicine. Developed first by the Swedish scientists Nils Löfgren and Bengt Lundqvist in 1943, it was marketed in 1948 after extensive tests. Its large success is largely attributed to its comparatively low neurotoxicity. Abraxis Pharmaceutical Products markets Lignocaine under the brand names of Xylocaine and Xylocard.
Jmol 3D structural views of molecule:
Uses
Lignocaine is a common local anaesthetic, injected as a dental anaesthetic and in minor surgery. It can also come in the form of an ointment as an antiarrhythmic to relieve itching, burning and pain from skin inflammations.
Side effects
It is rare to have any side effects caused by Lignocaine; however, allergic reactions can occur. Toxicity occurs for two reasons: either unintended intravascular administration, or administration of an excessive dose.
When excessive quantities of Lignocaine are used, the central nervous system and cardiovascular system may be affected. CNS effects may include CNS excitation such as nervousness, tingling around the mouth, tremors, blurred vision, seizure, followed by depression - drowsiness, loss of consciousness, respiratory depression and apnea. Cardiovascular effects include hypotension, bradycardia, arrhythmia, and cardiac arrest.
When Lignocaine is used as a local anaesthetic for regional nerve blocks, the plasma levels are usually at about 3-5 mcg/mL. Toxic effects can be detected when the plasma level is at 6 mcg/mL, but are common when the plasma levels exceed 10 mcg/mL. Although the quantity of dose is the major factor, there are several others that modulate the degree of toxicity. These include the relative vascularity of the injection site, and coincidentally the speed of the injection of the anaesthetic. Lignocaine is hepatically metabolized; this will increase the chance of liver dysfunction, which will in turn increase the risk of toxic effects. Being bound within a protein, when low protein states are encountered there is also a possibility that the toxicity will increase. The biological process of ‘acidosis’ increases the risk of toxicity, as it favours the dissociation of Lignocaine from the plasma proteins. Further medication can lead to interactions with anaesthetic, thus leading to increased toxicity as the level of Lignocaine is affected. Common medications with a chance of such reaction include: cimetidine, ciprofloxacin, clonidine, phenytoin, and beta-blockers such as propranolol, metoprolol, and nadolol.
How does it work?
Lidocaine works by changing the depolarisation in neurons. It achieves this by preventing the voltage gated sodium ion channel in the neuron’s axon cell membrane from functioning. When a threshold number of channels are blocked, the membrane will no longer be able to depolarise, therefore be unable to transmit an action potential. This inability leads to the molecule’s anaesthetic effect.
Potency of Lignocaine
Substituting the amide nitrogen of Lignocaine with an aminoethyl group increased the potency and improved the therapeutic index over the corresponding secondary amides when tested in mice.
References
- McMaster, P. D., E. W. Byrnes, et al. (1981). "New antiarrhythmic agents. 5. .alpha.-Aminoaceto-2,6-xylidides with functionalized amide alkyl substituents." J. Med. Chem. 24(1): 53-58. DOI: 10.1021/jm00133a012
