It07:Lactose
Introduction
Lactose is the most important carbohydrate in milk for most species (including humans). It is a carbohydrate found in milk and every newborn mammal consumes this in significant amounts. It is biosynthesised in the mammary gland and is industrially produced from bovine milk. It is also known as milk sugar. Lactose is extensively used in the pharmaceutical industry because it is inert, non-toxic and in-expensive.
| Lactose | |
|---|---|
| Lactose | |
| IUPAC Systematic name | |
| D-Glucose, 4-o-β-D-galactopyranosyl | |
| Other name | |
| {{{OtherName}}} | |
| Indentifiers | |
| ATC Code | |
| CAS number | {{{CASNo}}} |
| PubChem (CID) | [1] |
| SMILES | in nowiki script code '<' nowiki'>' insert SMILE here'</'nowiki'>' surround in nowiki script code '<' nowiki'>' insert SMILE here'</'nowiki'>' |
| Chemical Data | |
| Molecular formula | e.g.(C12H22O11) |
| Molar mass | 342 g/mol |
| Pharmacokinetic Data | |
| Bioavailability | |
| Protein Binding | {{{Protein_binding}}} |
| Metabolism | |
| Half life | |
| Excretion | |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | |
3D Model of Lactose
Sibutramine |
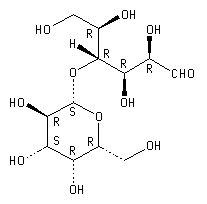
Structure of Lactose
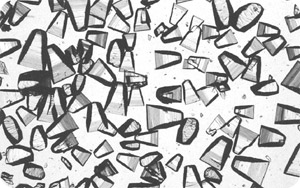
Lactose is present in two isomeric forms in milk - an α and β form. Both forms, however, interconvert frequently in a process called mutarotation. In solution the α and β forms strive for equilibrium commonly resulting in a ratio of α-lactose to β-lactose of 40:60. In its solid state it can exist either as a crystalline solid or amorphous. Crystalline lactose has two isomeric monohydrate forms which are both highly ordered arrangements. Amorphous lactose, however, lack this ordered structure with the lactose molecules arranged in a much more disordered way. Lactose monohydrate crystals are shown below - they are very brittle and hard.
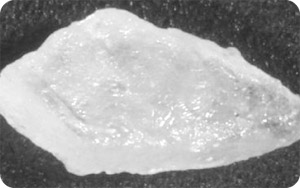
β-lactose is produced when highly concentrated lactose solutions are crystallised at temperatures above 93.5°C. β-lactose does not contain crystal water and it is much softer than α-lactose. β-lactose crystals are shown below:
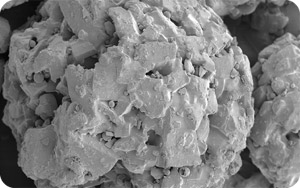
Amorphous lactose is produced by rapidly drying a highly concentrated solution of lactose. Amorphous lactose contains both isomeric forms of lactose - α and β forms.
Solubility
Lactose is soluble in water but is it is not as soluble as other common sugars. β-lactose is more soluble than α-lactose but the eventual solobility of both forms is equal because of the mutarotation phenomenan which ensures that equilibrium is established.
Synthesis
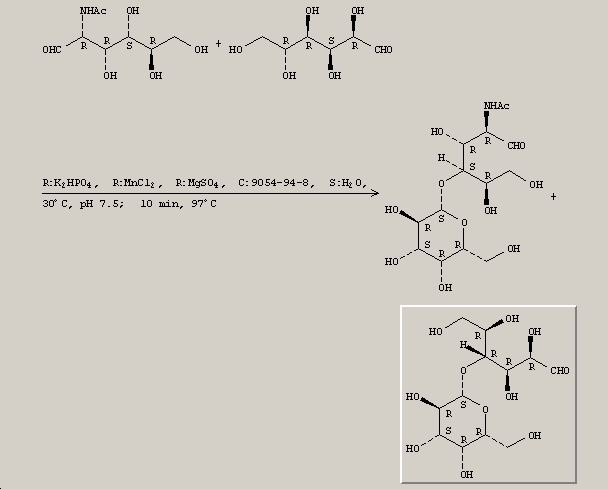
Below is the synthesis of lactose[1].
Uses
Lactose alpha monohydrate products can be used for capsules and sachets. Lactose can also be used as an excipient for pellets because of the fact that it is inert, cheap and non-toxic. Milled Lactose products can be used for wet granulation as-well as direct compression.
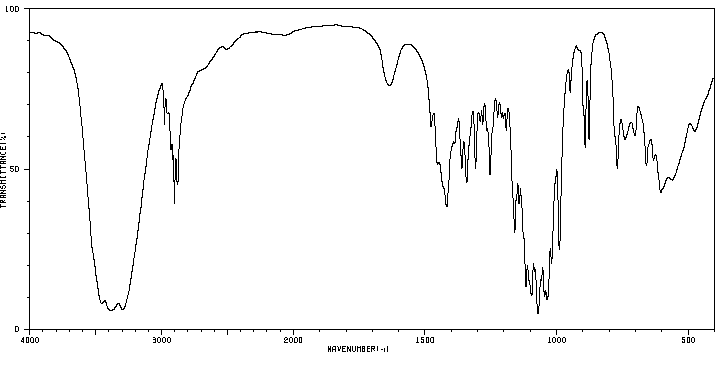
Infrared Spectrum of Lactose
References
- ↑ Metabolic engineering of Agrobacterium sp. for UDP-galactose regeneration and oligosaccharide synthesis. Ruffing, Anne; Mao, Zichao; Ruizhen Chen, Rachel. School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, Atlanta, GA, USA. Metabolic Engineering (2006), 8(5), 465-473.
2. http://www.lactose.com/index.html