It07:Lactic acid
| It07:Lactic acid | |
|---|---|
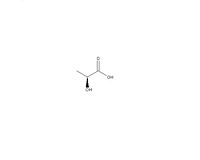
| |
| General | |
| IUPAC Systematic name | 2-hydroxypropanoic acid |
| Other names | Milk acid |
| Molecular formula | C3H6O3 |
| SMILES | C[C@H](O)C(O)=O |
| CAS number | 50-21-5 (L): 79-33-4 (D): 10326-41-7 (D/L): 598-82-3 |
| Molar mass | 90.08 gmol-1 |
| Properties | |
| Acidity (pKa) | 3.85 |
| Boiling point | 122 °C@12mmHg |
| Melting point | (L): 53 °C (D): 53 °C (D/L): 16.8 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Overview
Lactic acid, also known as milk acid, is a chemical compound which is very important in the biochemical process. It’s a carboxylic acid as shown in the template having a molecular formula C3H6O3. There’s a hydroxyl group next to the carboxyl group, which makes it becomes an alpha hydroxyl acid, known as AHA. When it is placed in the solution, the proton can be lost from the acidic group, generating the lactate ion CH3CH(OH)COO-. Furthermore, it’s hygroscopic as it’s miscible with either ethanol or water.
There’s a chiral centre in the lactic acid which generates two optical isomers. One is known as L-(+)-lactic acid or (S)-lactic acid. On the other hand, its mirror image, which is known as D-(-)-lactic acid or (R)-lactic acid. The L-(+)-Lactic acid is an important isomer in biology.
Lactic acid is made by a process called fermentation which releases energy from carbohydrate without requiring any oxygen. Lectate is one of the maim components of Ringer's lactate or lactated Ringer’s solution in medicine. This intravenous liquid which transfers the liquid substances into the vein, consists of potassium and sodium cations, with also chloride and lactate anions. In order for the concentration to be isotonic comparing to human blood, this liquid is in the solution with distilled water. It’s often used as the fluid resucitation after lossing of blood by burn injury or a surgery.
|
Cyclopentasiloxane |
Relationship between lacate and exercise
When performing some power-intensive exercises like sprinting, with a high demand of the energy, the lactate is generated a lot faster than the removal of it by the tissues. Thus the lactate concentration starts to rise. Due to the regeneration of nicotinamide adenine dinucleotide (NAD+), the energy production can be maintained and therefore the exercise can be continued. The increase in the lactate produced can be removed in several ways, e.g. by a process of gluconeogenesis in the liver to convert to glucose through the Cori cycle.
Lactic acid as a polymer
Lactic acid is commonly used as a monomer to produce polylactic acid (PLA) which is then turned to biodegradable plastic. The old conventional plastic made from petroleum oil is less applicable as there’s a higher emission of CO2 which causes global warming. Hence this biodegradable plastic is worth to substitute the conventional plastic.
Usage of lactic acid in foods
Normally lactic acis is found in some sour milk products like yogart, cottage cheese and kefir. In addition, lactic acis could also be found in different processed food. It can be either as a pH adjusting ingredient or as a preservative.
Spectra

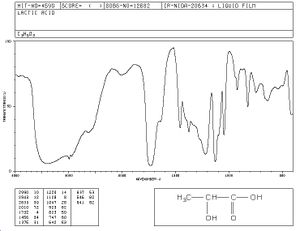
Reference
- http://en.wikipedia.org/wiki/Lactic_acid
- SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 7th December 2007)
