It07:Jacobsen
Jacobsen's Epoxidation Catalyst
The Jacobsen's catalyst, which was awarded the Fluka Prize "Reagent of the Year" in 1994, is an important catalyst widely used in the Jacobsen-Katsuki Epoxidation. This catalyst consists of a chiral salen ligand complexed with Manganese (III). It has the ability to convert cis-substituted olefins into chiral epoxides with high enantiomeric selectivity (usually with enantiomeric excesses of more than 90%, and sometimes even up to 98%!). It's also widely used in industry because only a few percent of the catalyst is needed, and common household bleach can be used as an oxidant. The Jacobsen catalyst could then be recycled and regenerated, so less chemical waste is produced.
Unlike many modern organometallic reagents (which are toxic, carcinogenic, etc), the Jacobsen's Catalyst is relatively safe and easy to use (even by organic students beginners). It is stable in air and water and is typically used only in small amounts (0.25–10 mol %) with sodium hypochlorite (bleach) as it is an ultimate oxidant. The catalyst is commercially available, but its preparation is simple and easy enough to understand.
2D Structure of the Molecule
| It07:Jacobsen | ||||||
|---|---|---|---|---|---|---|
| ||||||
| General | ||||||
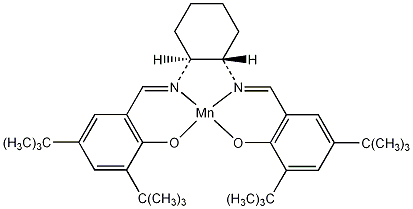
| Systematic name | N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine]manganese(III)]chloride 1 | |||||
| Other names | Mn Salen Ligand
Jacobsen's Epoxidation Catalyst | |||||
| Molecular formula | C36H52ClMnN2O2 | |||||
| SMILES | Cl[Mn]123OC(C(C(C)(C)C)=CC(C(C)(C)C)=C5)=C5C=[N@]1[C@](CCCC6)([H])[C@@]6([H])[N@]2=CC(C=C(C(C)(C)C)C=C4C(C)(C)C)=C4O3 | |||||
| Molar mass | 635.21208 gmol-1 | |||||
| Appearance | Dark brown powder | |||||
| CAS number | 138124-32-0 | |||||
| Properties | ||||||
| Density & phase | Not Available | |||||
| Solubility in water | Miscible in water | |||||
| Melting point | 330-332 oC | |||||
| Boiling point | Not Available | |||||
| Acidity (pKa) | Not Available | |||||
| Basicity (pKb) | Not Available | |||||
| Chiral rotation [α]D | {{{Rotation}}}° | |||||
| Viscosity | Not Available | |||||
| Structure | ||||||
| Molecular shape | {{{Mol_Shape}}} | |||||
| Coordination geometry |
{{{Coordination}}} | |||||
| Crystal structure | {{{Crystal_Structure}}} | |||||
| Dipole moment | {{{DM}}} D | |||||
| Hazards | ||||||
| MSDS | External MSDS | |||||
| Main hazards | Harmful by skin contact or inhalation | |||||
| NFPA 704 | {{{NFPA}}} | |||||
| Flash point | N/A | |||||
| R/S statement | R: {{{R-S}}} S: ? | |||||
| RTECS number | {{{RTECS}}} | |||||
| Supplementary data page | ||||||
| Structure and properties |
n, εr, etc. | |||||
| Thermodynamic data |
Phase behaviour Solid, liquid, gas | |||||
| Spectral data | UV, IR, NMR, MS | |||||
| Related compounds | ||||||
| Other anions | {{{Other_anion}}} | |||||
| Other cations | {{{Ohter_cation}}} | |||||
| Related compounds | {{{Relative_Compounds}}} | |||||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||||
The Jacobsen epoxidation catalyst is actually a molecule with the IUPAC name, N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine]manganese(III)]chloride 1 and has the following structure:
The first Salen catalyst introduced by Jacobsen was:
This then undergoes change to become the Jacobsen catalyst we all know and love today. It is a convenient tool and its now most widely used to synthesize the key side chain in the anti-tumour drug, Taxol.
The catalyst is produced in wide-scale quantities. It is quite simple to prepare this catalyst for a large-scale production. Its advantage is that only a few percent of the catalyst is used while using the common, low-cost everyday bleach. This produces less chemical waste, which is an important aspect in industry.
Jacobsen Catalyst Synthesis
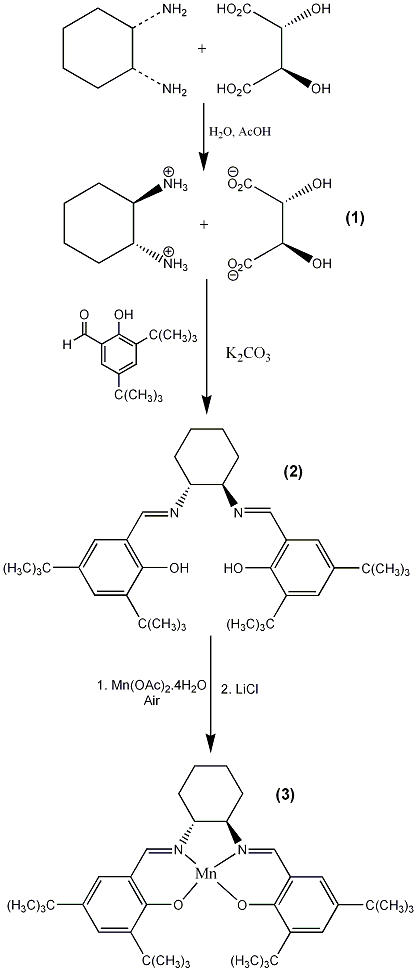
In the first step, 1,2-diaminocyclohexane is purified and resolved by crystallization of the salt formed with L-tartaric acid. As the hot reaction mixture cools, product crystallizes from the reaction mixture. The crude product is collected and recrystallized from water.
The diimine 2 (aka “Jacobsen’s ligand”) is formed by adding 3,5-di-tert-butyl-2-hydroxybenzaldehyde (dissolved in hot ethanol) to a refluxing solution of diaminocyclohexane salt 1 and potassium carbonate dissolved in a mixture of ethanol and water. After formation of the diimine, the reaction mixture is cooled in an ice bath. The crude solid product is then collected by vacuum filtration and dissolved in dichloromethane and washed with water and brine. Drying with sodium sulfate and evaporation of the solvent affords the diimine 2 as a yellow solid.
In the final step of the synthesis of Jacobsen’s catalyst (3), manganese(II) acetate is added to a refluxing solution of the diimine 2 in ethanol. Air is then bubbled through the reaction mixture to oxidize the manganese, producing a dark brown solution. The reaction is monitored by thin-layer chromatography and after the ligand has disappeared the air is discontinued and lithium chloride is added. The solvent is removed using a rotary evaporator and the residue dissolved in dichloromethane then washed with water and saturated sodium chloride (brine). After the dichloromethane layer is dried (Na2SO4), heptane is added and the dichloromethane (but not the heptane) is removed via rotary evaporation. The resulting dark brown solid is collected by vacuum filtration and allowed to dry in air.
Hazards
Occasionally fumes can be observed propagating from the top of the condenser when air is bubbled through the refluxing reaction mixture during production of Jacobsen’s catalyst. Therefore, this procedure should be done under a fumehood. Deuterochloroform and aromatic epoxides are suspected carcinogenics and should be handled with proper care.
The Jacobsen-Katsuki Epoxidation
The reaction scheme for the Jacobsen-Katsuki epoxidation is as follows:
Reaction Scheme 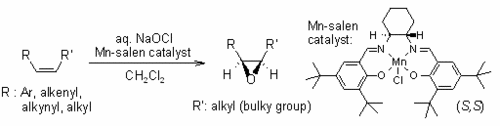
Mechanism
The mechanistic route for this reaction has two related but distinct aspects: the mode of oxygen transfer and the method of chiral induction. Since this is quite a new substance, neither aspect is fully understood.
Mode of oxygen transfer
The oxygen transfer occurs by a two-step catalytic cycle (see diagram below). In the first step, the oxidant (in this case, bleach) transfers oxygen to the MnIII catalyst in such a way that the oxygen orientates itself in a site normal to the Salen ligand. The Mn is thus oxidised from a +3 oxidation state to a +5 oxidation state. The activated oxygen is then delivered to the alkene to form an epoxide. This cycle is supported by observations that several oxidants have been used with identical selectivity, as opposed to assisted transfer by a complex aggregate. This reaction consumes stoichiometric quantities of the oxidant during the process of converting an alkene to an epoxide. The related salen complex then shows incorporation of oxygen from in situ labelled water, so this does not occur by exchange with the oxidant or epoxide.
Two-step catalytic cycle: 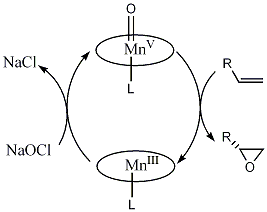
References
- T. Linker (Angew. Chem. Int. Ed., 1997, 36, 2060)
- Hanson, John. J. Chem. Educ. 2001 78 1266.
- http://www.organic-chemistry.org/namedreactions/jacobsen-katsuki-epoxidation.shtm
- http://www.ch.ic.ac.uk/ectoc/echet96/hyperwave/0000013f.html
- http://www.chemexper.com
- http://www.sigmaaldrich.com